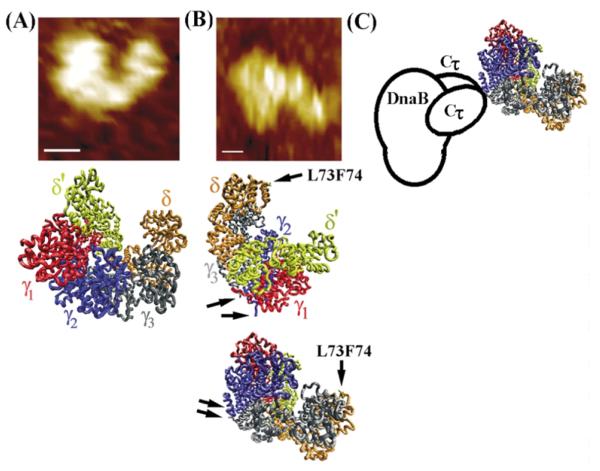
Figure 8.
The architecture of the τ–DnaB complex. (A) The shape of the B. subtilis τ protein resembles the E. coli clamp–loader complex. Comparison of the AFM topography image of τ (top) with the crystal structure of the clamp– loader complex (middle). (B) The architecture of the complex based upon the AFM topography image shown (top). Two possible orientations of the clamp-loader complex are shown. Arrows indicate the C-termini of the black and blue subunits (equivalent to τ1 and τ2, respectively) and the L73, F74 residues of δ that interact with the β clamp. (C) A model of the τ-DnaB complex. The clamp-loader interacts with the helicase via two Cτ domains of two τ subunits.