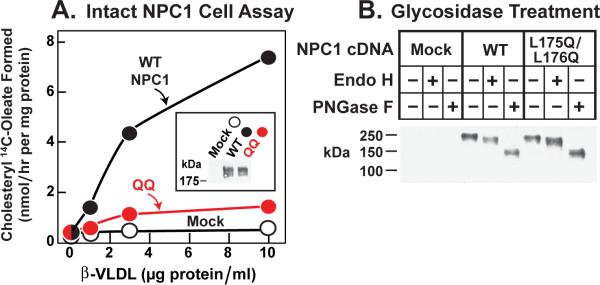
Figure 5. Failure of Mutant L175Q/L176Q Version of Full-length NPC1 to Rescue Cholesteryl Ester Formation in NPC1-defective Hamster Cells.
(A) Cholesterol esterification in response to ß-VLDL. NPC1-deficient CHO 4-4-19 cells were transfected on day 1 with 2 μg pcDNA3.1 (mock), WT pCMV-NPC1-His8-FLAG, or its mutant version (L175Q/L176Q) as described in Experimental Procedures. Five hr after transfection, the medium was switched to medium A containing 5% newborn calf lipoprotein-deficient serum. On day 2, the medium was switched to same medium containing 5 μM compactin and 50 μM sodium mevalonate. After 16 hr, fresh medium containing 50 μM compactin, 50 μM sodium mevalonate, and the indicated concentration of ß-VLDL was added. After 5 hr at 37°C, each monolayer was pulse-labeled for 1 hr with 0.2 mM sodium [14C]oleate (7433 dpm/nmol). The cells were then harvested for measurement of their content of cholesteryl [14C]oleate and [14C]triglycerides as described in Experimental Procedures. Each value is the average of duplicate incubations. The content of [14C]triglycerides for mock, WT, and mutant NPC1 transfected cells incubated with 10 μg/ml ß-VLDL was 201, 222, and 129 nmol/hr per mg protein, respectively. Inset shows an immunoblot of whole cell extracts from the various transfected cells probed with anti-FLAG antibody as described in Experimental Procedures.
(B) Deglycosidase treatment. NPC1-deficient CHO cells were transfected with the indicated plasmid as in (A). Five hr after transfection, the medium was switched to medium A with 5% FCS. Two days later, cells were harvested, and their solubilized membranes were treated with glycosidase EndoH or PNGaseF and then subjected to immunoblot analysis with anti-FLAG antibody as described in Experimental Procedures.
(A and B) Filters were exposed to film for ~10 sec at room temperature.