Figure 1.
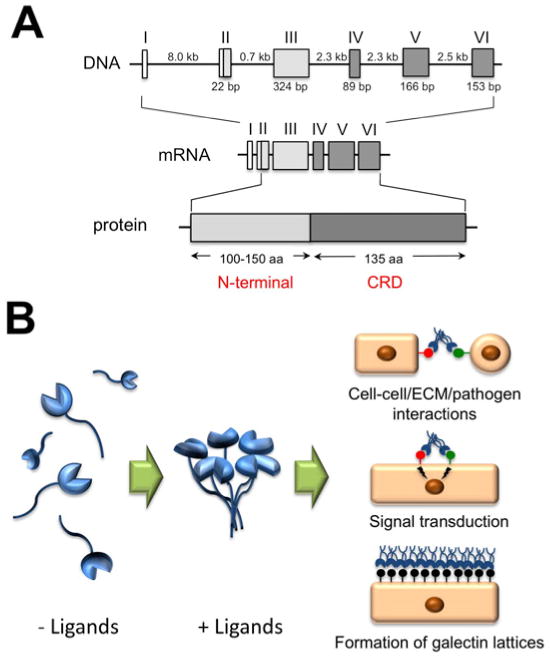
A. Genomic organization and protein structure of galectin-3. The Roman numerals indicate the position of the exons. The size of introns and polypeptide coding sequences are given in kb and bp, respectively. Galectin-3 consists of an N-terminal domain, 100-150 amino acids according to species of origin, and a C-terminal domain of about 135 amino acids, containing one carbohydrate recognition domain (CRD). B. Galectin-3 assembles in pentamer formation in the presence of multivalent ligands, in a process mediated through the N-terminal domain. At the cell surface, galectin-3 participates in cell-cell and cell-extracellular matrix (ECM) interactions, receptor clustering and signal transduction, and in the formation of glycoprotein-galectin lattices.
