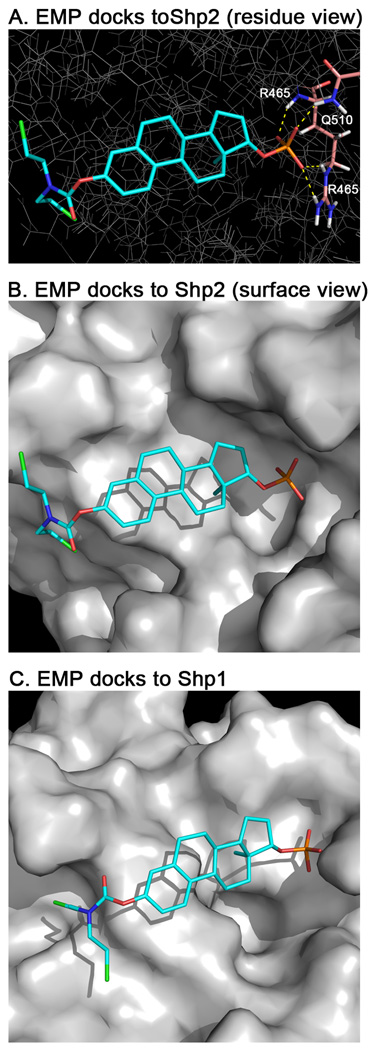
Fig. 3.
Docking of estramustine phosphate (EMP) to Shp2 and Shp1. EMP was docked into the catalytic site of Shp2 and Shp1 using GLIDE 5.6. (A) Stick representation of the lowest energy binding pose of EMP in Shp2. Potential hydrogen bonds are indicated as dashed-yellow lines showing interactions between the phosphate moiety and residues R465 (3 bonds) and Q510 (1 bond). (B) Water accessible surface showing the binding pocket. The binding pose of EMP fits into the deep pocket where the phosphate moiety can interact with the positive electrostatics of the region. (C) Water accessible surface of Shp1 with electrostatics. As with (B) the binding pose of EMP in Shp1 is shown. This pocket is much shallower and that may contribute to EMP’s inferior inhibition of Shp1 compared to Shp2.