Figure 1.
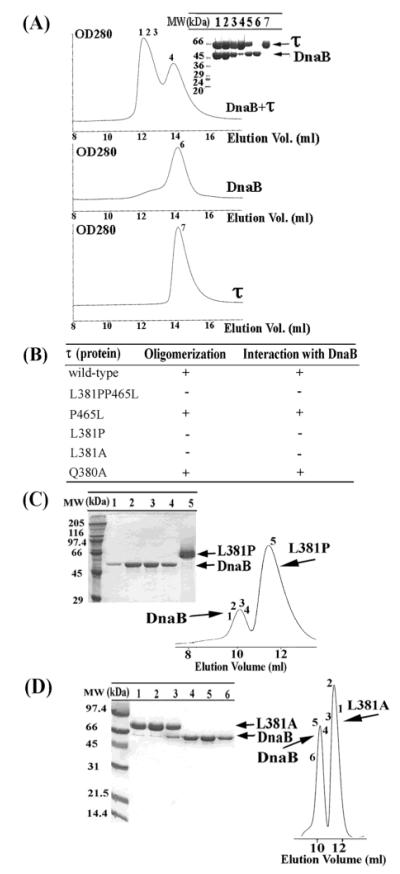
Mutations of τ that affect binding to DnaB. (A) Formation of a stable complex between the BstDnaB and the B. subtilis τ proteins. The DnaB-τ complex was resolved by gel filtration through a Superose 6 column. Samples from peaks (indicated by numbers) were analyzed by SDS–PAGE. The numbering of lanes corresponds to the numbering shown in gel filtration profiles. Lane 5 is a control lane, showing a mixture of purified τ and DnaB proteins in a 2:3 molar ratio (referring to monomers), for comparison. (B) A summary of the apparent oligomerization and DnaB-binding properties of τ mutants. A plus sign indicates apparent oligomerization and DnaB-binding properties similar to wt τ, whereas a negative sign indicates a defect. (C) Examining the L381P–BstDnaB interaction by gel filtration. A mixture of the L381P mutant protein and DnaB was resolved by gel filtration through a Superdex S-200 column. Samples, indicated by numbers, were analyzed by SDS–PAGE. The numbering of lanes corresponds to the numbers shown in gel filtration profiles. L381P and DnaB proteins are indicated by arrows. No stable interaction was detected. (D) Examining the L381A–BstDnaB interaction by gel filtration. The same experiment using the L381A mutant. Again, no stable interaction was detected.