Summary
To identify JAK/STAT signaling inhibitors, we performed a cell-based high throughput screening using a plant extract library and identified Nb-(α-hydroxynaphthoyl)serotonin called MS-1020 as a novel JAK3 inhibitor. MS-1020 potently inhibited persistently-active STAT3 in a cell type-specific manner. Upon further examination, we found that MS-1020 selectively blocks constitutively-active JAK3. MS-1020 consistently suppressed IL-2-induced JAK3/STAT5 signaling but not prolactin-induced JAK2/STAT5 signaling. Furthermore, MS-1020 affected cell viability only in cancer cells harboring persistently-active JAK3/STATs, and in vitro kinase assays showed MS-1020 binds directly with JAK3, blocking its catalytic activity. Therefore, our study suggested that this reagent selectively inhibits JAK3 and subsequently leads to a block in STAT signaling. Finally, we showed MS-1020 decreases cell survival by inducing apoptosis via down-regulating anti-apoptotic gene expression. Our study suggests that MS-1020 may have therapeutic potential in the treatment of cancers harboring aberrant JAK3 signaling.
Keywords: JAK/STAT, cancer, small molecule inhibitor, plant extracts, cell-based high throughput screening, Drosophila
Introduction
In the early 1990s, the JAK/STAT signal transduction cascade was first discovered by studies on gene induction by interferons (IFNs) (Schindler et al, 1992; Velazquez et al, 1992; Shuai et al, 1992, 1993a, 1993b, 1994; Watling et al, 1993). In mammals, four JAK and seven STAT genes have been identified and more than forty different cytokines and growth factors have been shown to activate specific combinations of JAK or STAT proteins (Schindler et al, 2007). The analysis of humans and mice lacking JAK and STAT function revealed that these molecules are required for a wide variety of biological processes, including the regulation of immune response and hematopoietic progenitor cells (Nosaka et al, 1995; Park et al, 1995; Thomis et al, 1995; Meraz et al, 1996; Neubauer et al, 1998).
Recent extensive surveys of primary tumors and cell lines derived from tumors indicate that inappropriate activation of JAK/STAT signaling occurs with high frequency in human cancers (Darnell, 2005). In particular, STAT3 and STAT5 are often found to be hyper-activated in leukemia and lymphoma, as well as solid tumors such as breast cancer (Yu & Jove, 2004; Klampfer, 2006). Persistently-active JAK kinases are also found to be expressed in a variety of blood malignancies, and the activating alleles of JAK1, JAK2, and JAK3 were identified in patients with those cancers. Eighteen percent of adult T-cell acute lymphoid leukemia patients carry somatic JAK1 mutations (Flex et al, 2008), and a majority of patients with myeloproliferative diseases, including polycythemia vera (PV) harbor JAK2 mutation (Baxter et al. 2005; James et al. 2005; Levine et al. 2005; Jones et al. 2009; Kilpivaara et al. 2009; Levine, 2009; Olcaydu et al. 2009). This JAK2 mutation, encoding a V617F substitution, promotes JAK2 catalytic activation and cytokine-independent signaling (Kota et al, 2008). Somatic mutations of JAK3 were also identified in a minority of acute megakaryoblastic leukemia (AMKL) patients both in Down syndrome children and non-Down syndrome adults (Walters et al, 2006), and in a patient with acute lymphoblastic leukemia (Mullighan et al., 2009). These observations make JAK and/or STAT proteins attractive therapeutic targets for human cancers.
To identify JAK/STAT signaling inhibitors, we performed a cell-based high throughput screening, using a library of natural products extracted from various plant species and a cultured Drosophila cell line stably expressing a STAT reporter gene. In this study, we identified Nb-(α-hydroxynaphthoyl)serotonin (MS-1020) that blocks STAT reporter activity in Drosophila cells and selectively inhibits JAK3 signaling in mammalian cancer cells. Since in vitro kinase assays showed that MS-1020 directly blocks JAK3 catalytic activity, MS-1020 likely inhibits JAK3 activity and subsequently blocks downstream STAT signaling. We also demonstrate that MS-1020 decreases cell viability by inducing programmed cell death via down-regulating the expression of anti-apoptotic genes, known STAT downstream targets.
Materials and methods
Cell lines and culture conditions, and luciferase reporter constructs
Parental macrophage-like Drosophila Schneider (S2-NP) cells were maintained in Schneider's Drosophila medium supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin in an incubator at 25°C. S2-NP-STAT92E cells that stably express both the 10×STAT92E-firefly luciferase reporter gene and the RNA polymerase III (Pol III)-Renilla luciferase gene were also grown in the same medium but supplemented with 500 μg/mL G418. The 10×STAT92E-firefly luciferase reporter gene was generated by placing five tandem repeats of a 441-bp fragment (base pairs 89,227-89,667 of scaffold AC093048) in the enhancer of the SOCS36E gene upstream of a minimal heat-shock promoter-driven cDNA encoding the firefly luciferase gene (Baeg et al., 2005). The PolIII-Renilla luciferase control construct was generated by PCR amplification of a fragment (base pairs 43,224-43,389 of scaffold AE003823) of the promoter of the D. melanogatser RNA PolIII 128 subunit (RplIII128) and ligation of this fragment into pRL-null (Nybakken et al., 2005). Hodgkin's lymphoma L540 and HLDM-2 cells were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) and grown in RPMI 1640 containing 100 U/mL penicillin and 100 μg/mL streptomycin with 20% FBS. A breast cancer cell line MDA-MB-468 and a multiple myeloma cell line U266 were purchased from the American Type Culture Collection (Manassas, VA, USA) and maintained in DMEM containing 100 U/mL penicillin and 100 μg/mL streptomycin with 10% FBS. Rat pre-T lymphoma Nb2 cells were kindly provided by Dr. Charles Clevenger (Northwestern University) and grown in RPMI 1640 supplemented with 10% FBS, 2mM L-glutamine, 5 mmol/L HEPES, pH 7.4, 100 U/mL penicillin and 100 μg/mL streptomycin. Human cancer cell lines and rat Nb2 cells were cultured at 37°C in a humidified incubator containing 95% air and 5% CO2. Schneider's Drosophila medium, DMEM, RPMI 1640, fetal bovine serum (FBS), and penicillin/streptomycin were obtained from Invitrogen (Carlsbad, CA, USA).
Identification of natural products that inhibit JAK/STAT signaling in cultured Drosophila cells
To identify novel JAK/STAT signaling inhibitors, a cell-based high throughput screening was performed using a library of the 3,600 methanol extracts of various plant species grown in the Korean Peninsula which were obtained from the Plant Extract Bank in the Korea Research Institute of Bioscience and Biotechnology (http://www.pdrc.re.kr/index.asp). S2-NP-STAT92E cells were co-cultured for 24 hours with Upd (cytokine)-producing cells, which are parental S2-NP cells transiently transfected with actin promoter-driven Upd using an Effectene transfection reagent (Qiagen, Valencia, CA, USA), in the presence of plant extracts at the concentration of 300 μg/mL. The STAT92E reporter activity was quantified by measuring relative luciferase units (RLU), which equaled the ratio of the absolute activity of firefly luciferase to Renilla luciferase. The cytotoxicity effect of each plant extract was monitored by measuring Renilla luciferase activity, and those that resulted in more than 25% decrease in the activity compared with that of control were discarded and no longer considered hits. We performed the primary screen in duplicates, and identified the extract of Phragmites communis, Trin. that blocks STAT92E reporter activity in a dose-dependent manner.
Isolation of active compounds from Phragmites communis, Trin. extracts, and synthesis of MS-1020
The dried roots of Phragmites communis Trin. were extracted with methanol (MeOH) three times at room temperature. The MeOH extract was suspended in H2O, and extracted with n-hexane, ethyl acetate (EtOAc) and n-butanol sequentially. The EtOAc soluble fraction (PCE) showed ability to inhibit STAT92E reporter activity. All fractionation and separation steps were accompanied with biological assays. PCE was chromatographed on a reversed phase silica gel (RP-18) column, and eluted with MeOH-H2O mixture (MeOH 50% to 100%, v/v, step gradient) that produced three different fractions (PCE1 to PCE3). PCE1 was chromatographed on a silica gel column eluting with CHCl3-MeOH mixture (MeOH 5% to 100 %, v/v, step gradient) and afforded an active fraction (PCE1-3) from six sub-fractions (PCE1-1 to PCE1-6). PCE1-3 was again chromatographed on a RP-18 column eluting with MeOH-H2O mixture (MeOH 30% to 100% step gradient) and yielded seven sub-fractions (PCE1-3a to PCE1-3g). The active sub-fraction PCE1-3e was chromatographed on a silica gel column eluting with hexane-ethylacetate-methanol mixture (2/2/9, v/v/v) to give nine sub-fractions (PCE1-3e-1 to PCE1-3e-9). As active compounds responsible for JAK/STAT signaling inhibition, Nb-(p-coumaroyl)serotonin and Nb-(p-feruloyl)serotonin were isolated from the fractions of PCE1-3e-4 and PCE1-3e-6, respectively, using a preparative HPLC system (RP-18, elution: MeOH/H2O, 1/1, v/v). To obtain small molecules that show more potency at blocking JAK/STAT signaling, MS-1020, Nb-(α-hydroxynaphthoyl)serotonin was synthesized by the chemical reaction between 1-hydroxy-2-naphtoic acid and 1-hydroxybenzotriazole in N,N-dimethylformamide and the solution of serotonin hydrochloride, followed by extraction with ethyl acetate and purification using column chromatography.
Western blot analysis, cell viability assay, and apoptosis assay
Cell pellets were suspended in a lysis buffer containing 50 mM Tris-HCl, pH 7.4, 350 mM NaCl, 1% Triton X-100, 0.5% Nonidet P-40, 10% glycerol, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 1 mM phenylmethylsulphonyl fluoride (PMSF) and phosphatase inhibitor cocktails on ice. Whole-cell extracts were resolved on SDS-PAGE, transferred to nitrocellulose membrane, and probed with appropriate antibodies: phospho-JAK3 (Tyr980), JAK3, STAT3, STAT5 and Lyn were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and used at a dilution of 1:500∼1:2000. Antibodies specific for phospho-STAT3 (Tyr705), phospho-STAT5 (Tyr694), JAK1, phospho-JAK2 (Tyr1007/1008), JAK2, phospho-Tyk2 (Tyr1054/1055), Tyk2, phospho-Src (Tyr416), phospho-Lyn (Tyr507), phospho-Akt (Ser473), phospho-ERK1/2 (Thr202/Tyr204), phospho-EGFR (Tyr1068), PARP, caspase-3, Bcl-2, Bcl-xL, Mcl-1, survivin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology (Danvers, MA, USA) and used at a dilution of 1:1000∼1:2500. Phospho-JAK1 (Tyr1022/1023) antibody was obtained from upstate (Temecula, CA, USA) and used at a dilution of 1:1000. Membranes were blocked in 5% non-fat dry milk in Tris-buffered saline (pH 7.4) containing 0.1% Tween 20 (TBST) for 1 hour and subsequently incubated with primary antibodies diluted in TBST at 4°C for overnight. Membranes were then probed with horseradish peroxidase-conjugated secondary antibodies and then developed using Enhanced Chemiluminescence Reagent (GE Healthcare Bio-Sciences, Piscataway, NJ, USA).
For cell viability assay, L540 and HDLM-2 cells (5×104 cells/mL) were treated with either vehicle (DMSO) alone, MS-1020 at different concentrations, or the pan-JAK inhibitor AG490 (100 μmol/L) and incubated for the indicated time periods. Trypan blue exclusion assay was performed to count viable cells. For apoptosis assay, Terminal Transferase dUTP Nick End Labeling (TUNEL) assay was conducted as described (Kim et al, 2008). Briefly, L540 cells (1.0×106 cells /mL) were treated with either vehicle (DMSO) alone or MS-1020 at various concentrations ranging up to 50 μmol/L for 72 hours, stained using an APO-BRDU kit (Phoenix Flow Systems, Inc., San Diego, CA, USA), and subsequently subjected to Elite ESP flow cytometry (Coulter Inc., Miami, FL, USA).
To demonstrate that MS-1020-induced apoptosis in L540 cells resulted from reduced JAK3 activity, the effect of JAK3 siRNA treatment on the expression of anti-apoptotic genes was examined. Human JAK3 siRNA and scrambled siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). L540 cells were transfected by electroporation using an Amaxa Nucleofector (Lonza Walkerrsville Inc., Walkersville, MD, USA).
Purification of recombinant His-tagged STAT3α protein, and in vitro kinase assay
A full-length STAT3α cDNA was PCR-amplified using the primers; 5′-CACGGATCCGCCCAATGGAATCAGCTACAG-3′ and 5′-ATTAAGCTTCATGGGGGAGGTAGCGCACTC-3′ and a pcDNA-Myc-STAT3α plasmid as a template. The PCR products were sub-cloned into the pQE-30 expression vector (Qiagen, Valencia, CA, USA) using BamHI and Hind III restriction sites to make a pQE-30-His-tagged STAT3α plasmid. The E. coli. M15[pREP4] cells (Qiagen, Valencia, CA, USA) were transformed with the plasmid and cultured with 0.1 mmol/L isopropyl-beta-D-thiogalactopyranoside (IPTG). Recombinant His-tagged STAT3α was purified using the TALON Metal Affinity Resin Kit (Clontech, Mountain View, CA, USA), according to the manufacturer's protocol and used as a substrate for in vitro kinase assay.
For JAK kinase assay, L540 or HDLM-2 cells were lysed in a lysis buffer containing 20 mM Tris-HCl, pH 7.4, 500 mM NaCl, 0.25% Triton X-100, 1 mM EDTA, 1 mM EGTA, 10 mM β-glycerophosphate, 1 mM DTT, 300 μM Na3VO4, 1 mM phenylmethylsulphonyl fluoride (PMSF) and phosphatase inhibitor cocktails, and pre-cleared with protein A/G-sepharose for 2 hours at 4°C. The lysates were then incubated with anti-JAK2 or anti-JAK3 antibody for overnight at 4°C, and the immune complexes were precipitated by protein A/G-sepharose beads. The precipitates were washed with kinase buffer (25 mM Tris/HCl, pH 7.5, 20 mM β-glycerophosphate, 10 mM MgCl2, 2 mM DTT, 1 mM Na3VO4, and protease inhibitor cocktail). Kinase reaction was subsequently performed by the addition of either vehicle (DMSO) alone, MS-1020 at different concentrations or AG490, 2 μg His-tagged STAT3α proteins, and 2 μmol/Lol/L ATP (20 μmol/Lol/L ATP for competition experiments) for 30 minutes at 30°C. The reaction products were subjected to SDS-PAGE and probed with antibodies specific for phospho-STAT3, STAT3, JAK2, or JAK3.
Results
Identification of plant extracts that inhibit JAK/STAT signaling in cultured Drosophila cells
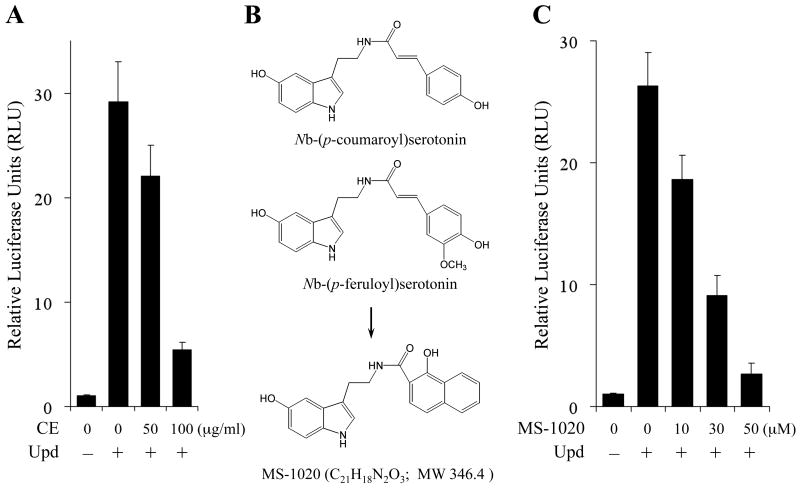
We previously showed that a cultured Drosophila cell line can be used as a useful tool to identify the small molecule inhibitors of JAK/STAT signaling, at least in part due to the reduced redundancy of JAK/STAT pathway core components in the Drosophila genome compared to those in mammalian genomes (Kim et al, 2008). The JAK/STAT pathway in Drosophila consists of only one JAK called Hop and one STAT called STAT92E. STAT92E is most similar to STAT3 and 5 (Zeidler et al, 2000), and is thought to regulate transcription in a manner similar to that observed by mammalian STATs (Arbouzova & Zeidler, 2006), thus making STAT92E a useful model to identify small molecules that inhibit JAK/STAT transcriptional output (Kim et al, 2008). To identify such molecules, we carried out a cell-based high throughput chemical screening using a library of 3,600 crude extracts from various plant species grown in the Korean Peninsula and a cultured Drosophila cell line that stably expresses both the STAT92E transcriptional reporter and the PolIII-Renilla gene (Kim et al. 2008). These cells were co-cultured for 24 hours with Upd (cytokine)-producing cells in the presence of the library of crude extracts at 300 μg/mL (see Materials and Methods). The reporter activity was quantified by measuring RLU. From the screening, we detected the inhibitory effects of products extracted from Phragmites communis, Trin. on the reporter activity (Fig 1A). These extracts blocked Upd-induced STAT92E transcriptional activity in a dose-dependent manner, but did not show any cytotoxicity up to 300 μg/mL which was determined by monitoring the activity of Renilla luciferase.
Fig 1.
MS-1020 inhibits STAT92E transcriptional activity in cultured Drosophila cells. (A) Crude extracts (CE) of Phragmites communis Trin. inhibit Upd (cytokine)-induced STAT92E reporter activity in a dose-dependent manner. Cultured Drosophila S2-NP-STAT92E cells expressing a STAT92E reporter gene were co-cultured for 24 hours with Upd-producing cells in the presence of Phragmites communis Trin. extracts at different concentrations. The STAT92E reporter activity was normalized as the ratio of firefly luciferase to Renilla. The reporter activity without Upd stimulation was set to 1. Results are shown as the mean of three independent experiments (± SD indicated by error bar). (B) The chemical structures of Nb-(p-coumaroyl)serotonin, Nb-(p-feruloyl)serotonin, and MS-1020. Nb-(p-coumaroyl)serotonin and Nb-(p-feruloyl)serotonin were isolated as active compounds responsible for JAK/STAT signaling inhibition, from the extracts of Phragmites communis Trin. A derivative, MS-1020 was chemically synthesized (see Materials and Methods). (C) Dose effect of MS-1020 on Upd-induced STAT92E reporter activity. S2-NP-STAT92E cells were co-cultured for 24 hours with Upd-producing cells in the presence of either vehicle (DMSO) alone or MS-1020 at various concentrations up to 50 μmol/Lol/L. Results are shown as the mean of three independent experiments (± SD indicated by error bar).
A preparative HPLC system was employed to isolate active compounds from this plant extract, and two compounds, Nb-(p-coumaroyl)serotonin and Nb-(p-feruloyl)serotonin were identified (see Materials and Methods; Fig 1B). Since the IC50 values of these two compounds were between 50∼70 μmol/Lol/L (data not shown), we attempted to synthesize the derivatives of these compounds to obtain small molecules that show better potency on inhibiting JAK/STAT signaling (see Materials and Methods). MS-1020, Nb-(α-hydroxynaphthoyl)serotonin was synthesized (Fig 1B), and this reagent potently blocked Upd-induced STAT92E signaling (Fig 1C). The treatment of 30 μmol/Lol/L MS-1020 reduced the reporter activity by more than 50%, whereas 50 μmol/Lol/L MS-1020 blocked STAT92E transcriptional activity back to the level observed with vehicle (DMSO) alone. Since tyrosine phosphorylation is a key step in STAT transcriptional activation on cytokine/receptor stimulation, we next assessed if MS-1020 inhibited tyrosine phosphorylated STAT92E levels. As expected, 50 μmol/Lol/L MS-1020 almost completely abrogated Upd-induced STAT92E phosphorylation (data not shown). These results suggest that MS-1020 is a novel inhibitor of JAK/STAT signaling in Drosophila.
MS-1020 inhibits STAT signaling in cancer cells with constitutive JAK3 activity
We next assessed if MS-1020 can also block STAT signaling in human cells. We first examined the effects of MS-1020 on the activity of STAT3, which is the most common form found in human cancers (Darnell, 2005). In these experiments, we used the Hodgkin's lymphoma cell lines, L540 and HLDM-2, and a breast cancer cell line MDA-MB-468 because these cell lines highly contain persistently-active STAT3. Phospho-STAT3 was detected using an antibody specific for phospho-STAT3Y705. MS-1020 showed ability to reduce tyrosine phosphorylated STAT3 levels in a dose-dependent manner in L540 cells (Fig 2A, lane 1). Treatment with 30 μmol/Lol/L MS-1020 abolished phosphorylated STAT3 levels by more than 70%, whereas total STAT3 levels remained unchanged at the concentrations up to 50 μmol/Lol/L (Fig 2A, lane 1 and 2). Interestingly, we found that treatment with 50 μmol/Lol/L MS-1020 failed to inhibit constitutively-active STAT3 in HDLM-2 and MDA-MB-468 cells (Fig 2B and C, lane 1). On the other hand, the pan-JAK inhibitor AG490 effectively suppressed constitutively-active STAT3 in all cell lines tested (Fig 2A-C, lane 1).
Fig 2.
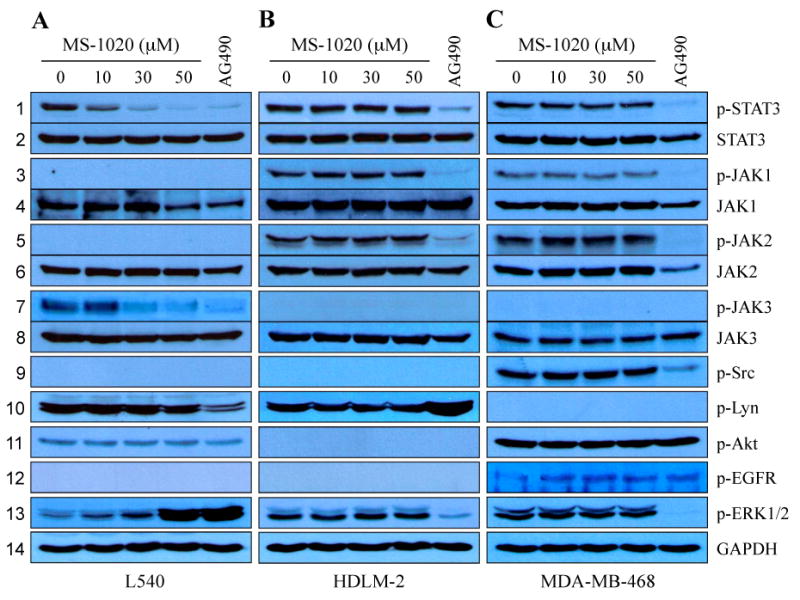
MS-1020 inhibits JAK3/STAT signaling. L540 (A), HDLM-2 (B) and MDA-MB-468 (C) cells were cultured for 24 hours in the presence of either vehicle (DMSO) alone, MS-1020 at different concentrations or the JAK kinase inhibitor AG490 (150 μmol/L). Whole- cell extracts were processed for Western blot analysis using antibodies specific for the molecules indicated. Treatment with MS-1020 inhibited constitutively-active STAT3 in a cell type-dependent manner (lane 1 and 2). Interestingly, MS-1020 effectively decreased phosphotyrosine JAK3 levels, and showed selectivity for JAK3 over other JAK kinases and oncogenic pathway components, such as Src, Lyn, Akt, EGFR and ERK1/2 (lanes 3-13), suggesting the selectivity of MS-1020 for JAK3. These results are consistent with the finding that MS-1020 abrogated persistently-active STAT3 in cells only with hyper-activated JAK3, such as L540 cells. GAPDH serves as a loading control.
To gain further insights into the mechanisms of MS-1020 on STAT3 inhibition, we next examined whether MS-1020 can affect the activity of JAK family members, which are key upstream regulators of STAT3 signaling (Levy & Darnell, 2002). We used phospho-specific JAK1, 2, and 3 antibodies. In L540 cells tyrosine phosphorylated JAK1 and JAK2 levels were below the levels of detection, whereas persistently-active JAK3 was strongly apparent. JAK3 activation was abrogated by MS-1020 treatment in a dose-dependent manner (Fig 2A, lanes 3-8). Phospho-JAK3 was almost completely suppressed at the 30 μmol/L concentration of MS-1020 that induced a dramatic reduction of phosphotyrosine STAT3 levels (Fig 2A, lane 1). Conversely, we found no inhibitory effects of MS-1020 at the concentrations up to 50 μmol/L in HDLM-2 and MDA-MB-468 cells, which contain constitutively-active forms of JAKs 1 and 2 but not 3 (Fig 2B and C, lanes 3-8). This observation is consistent with the finding that 50 μmol/L MS-1020 did not induce any significant reduction of STAT3 phosphorylation levels in HDLM-2 and MDA-MB-468 cells (Fig 2B and C, lane 1). In contrast, the pan-JAK inhibitor AG490 non-selectively inhibited the phosphorylation levels of all JAK kinases tested in those cells. We next examined the effects of MS-1020 on other JAK kinase member, Tyk2 (Velazquez et al. 1992). The U266 myeloma cells were pre-treated with MS-1020 and then activated by IFN-α for 30 minutes. While AG490 completely blocked IFN-α-induced Tyk2 phosphorylation, treatment with MS-1020 showed no effect on phosphorylated Tyk2 levels (Supplementary Fig 1).
STAT3Y705 activation has also been shown to be mediated by other oncogenic kinases, including some Src family tyrosine kinases (Parsons & Parsons, 2004). We first assessed if MS-1020 can inhibit Src kinases, we treated L540, HDLM-2 and MDA-MB-468 cells with up to 50 μmol/L MS-1020 and found that these concentrations did not affect phosphorylated levels of Src and Lyn (Fig 2, lane 9 and 10). We also examined the effects of MS-1020 on the activation of the serine/threonine kinase Akt and tyrosine kinase EGFR, and found that this reagent has no inhibitory effects on phospho-Akt and phospho-EGFR levels at the concentrations up to 50 μmol/L (Fig 2, lane 11 and 12). STAT activity has been reported to be regulated by Ras/Raf/MEK/ERK (Steelman et al, 2004; Diaz et al, 2006). We thus examined whether MS-1020 can affect ERK1/2 activation. The phosphorylation of ERK1/2 levels was not altered after the treatment of MS-1020 at the concentrations up to 50 μmol/L in both HDLM-2 and MDA-MB-468 cell lines (Fig 2B and C, lane 13). However, in L540 cells we detected the up-regulation of phospho-ERK1/2 levels in a dose-dependent manner (Fig 2A, lane 13). JAK signaling is required for G2-M transition with the inhibition of ERK1/2, and thus JAK inhibition lead to an increase in ERK1/2 phosphorylation levels in HL-60 myeloblastic leukemia cells (Reiterer & Yen, 2006). Therefore, our observation suggests that the up-regulation of ERK1/2 phosphorylation levels in MS-1020-treated L540 cells resulted from the disruption of G2-M transition. Nonetheless, our results strongly suggest that MS-1020 blocks STAT3 signaling through inhibiting upstream regulator JAK3.
MS-1020 blocks STAT signaling induced by IL-2 in rat T-lymphocyte Nb2 cells
JAK2 plays a pivotal role in signal transductions through the highly related receptors for cytokines and some hormones, including IL-3, Epo (erythropoietin), GM-CSF (granulocyte-macrophage colony-stimulating factor), PRL (prolactin), and growth hormone (Chilton & Hewetson, 2005; Pesu et al, 2008). In particular, PRL receptors are considered selective activators of JAK2 but not of JAK1, JAK3, or Tyk2 (Neilson et al, 2007). On the other hand, JAK3 is known to be activated through the association with only the shared common gamma chain (γc) of IL-2, IL-4, IL-9, IL-15 and IL-21 receptors (O'Shea et al, 2004; Pesu et al, 2005). In the rat pre-T lymphoma Nb2 cells, JAK2 undergoes rapid and transient tyrosine phosphorylation in response to PRL, whereas JAK3 becomes tyrosine phosphorylated upon IL-2 stimulation (Kirken et al, 1994; Rui et al, 1994). Therefore, Nb2 cells can serve as a practical cellular model for comparative studies of PRL- and IL-2-induced signal transduction (Kirken et al, 1994).
To assess if MS-1020 has selectivity for JAK3 inhibition, we examined the effects of this compound on STAT5 signaling induced by PRL or IL-2 stimulation in Nb2 cells. Cells were incubated in RPMI 1640 medium with 5% gelded horse serum and 1× ITS liquid media for starvation in the presence of MS-1020 for 16 hours, and then stimulated by PRL or IL-2 for 10 minutes. We used AG490, a pan-JAK inhibitor, as a control. While phosphorylated STAT5 was barely detected in cells without stimulation, we detected a dramatic increase in phosphorylated STAT5 levels in response to either PRL or IL-2 treatment (Fig 3A and B). As expected, AG490 non-selectively blocked the tyrosine phosphorylation of STAT5 induced by either PRL or IL-2 (Fig 3A and B). In contrast, MS-1020 treatment at the concentrations up to 50 μmol/L did not result in a significant reduction of PRL-induced STAT5 phosphorylation. In contrast, this compound efficiently inhibited IL-2-induced STAT5 phosphorylation via JAK3 in a dose-dependent manner. In fact, IL-2-induced STAT5 phosphorylation was almost undetectable at 50 μmol/L of MS-1020 (Fig 3B). These findings are consistent with our previous observation that MS-1020 selectively inhibits JAK3/STAT signaling (Fig 2).
Fig 3.
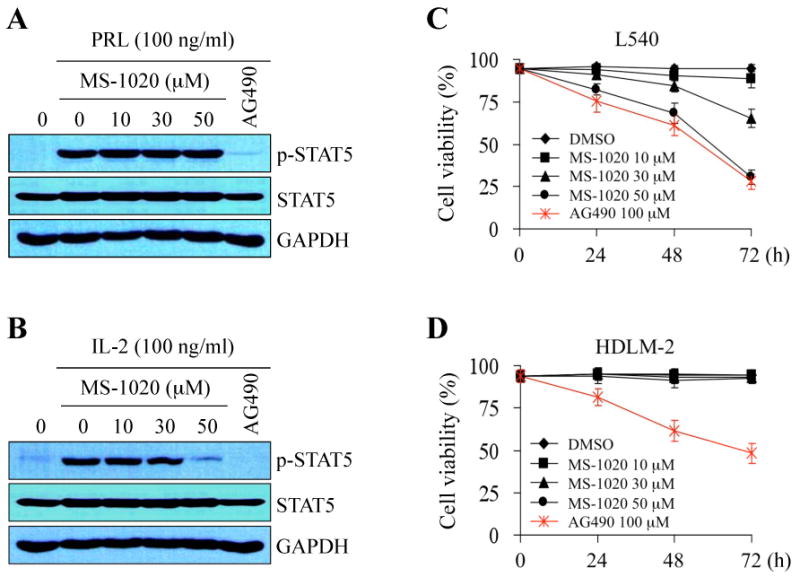
MS-1020 affects STAT signaling and cell viability dependent of JAK3 activation. (A and B) Effects of MS-1020 on phosphotyrosine STAT5 induced by either PRL (prolactin) (A) or IL-2 (B) were investigated. Rat pre-T lymphoma Nb2 cells, which can be used for comparative studies of PRL- and IL-2-induced signal transduction, were starved for 16 hours in the presence of either vehicle (DMSO) alone, MS-1020 at different concentrations or the JAK kinase inhibitor AG490 (150 μmol/L). Cells were subsequently stimulated by PRL (100 ng/mL) or IL-2 (100 ng/mL) for 10 minutes. Antibodies specific for phospho-STAT5 and STAT5 were used for Western blot analysis. Note that AG490, a pan-JAK inhibitor, non-selectively blocked STAT5 phosphorylation induced by PRL or IL-2. In contrast, MS-1020 selectively inhibited phosphotyrosine STAT5 induced only by IL-2 but not PRL. GAPDH serves as a loading control. (C and D) MS-1020 affected cell viability in cells only with aberrant JAK3/STAT signaling. L540 (C) and HLDM-2 (D) cells were treated with either vehicle (DMSO) alone, MS-1020 at different concentrations or the JAK kinase inhibitor AG490 (100 μmol/L), and incubated for the indicated time periods. Trypan blue exclusion assay was performed to count viable cells. While AG490 affected viability in both cells, treatment with MS-1020 decreased viability only in L540 cells that express constitutively-active JAK3/STAT (see Fig 2). Results are shown as the mean of three independent experiments (± SD indicated by error bar).
MS-1020 selectively affects cell viability harboring constitutively-active JAK3
Since the inhibition of JAK/STAT signaling was reported to decrease cancer cell survival (Epling-Burnette et al, 2001; Mahboubi et al, 2001; Battle & Frank, 2002) and MS-1020 is considered a selective JAK3/STAT signaling inhibitor, we hypothesized that treatment with MS-1020 will affect cell viability only in cancer cells with constitutive JAK3/STAT activity. To test this hypothesis, we examined the effects of MS-1020 on cell survival in Hodgkin's lymphoma L540 and HDLM-2 cells that express persistently-active JAK3/STATs and JAK1/JAK2/STATs, respectively (Fig 2A and B). Cells were treated with either vehicle (DMSO) alone, MS-1020 at various concentrations, or AG490 as a control. While AG490 decreased cell survival in both cell lines, MS-1020 promoted cell death in a time- and dose-dependent manner only in L540 cells, which express constitutive-active JAK3, but not HDLM-2 cells, which do not (Fig 3C and D).
MS-1020 directly blocks JAK3 kinase activity
To obtain more insight into the mechanism by which MS-1020 inhibits JAK3, we next performed in vitro kinase assays using recombinant His-tagged STAT3α proteins as a substrate. We immunoprecipitated JAK2 and JAK3 from HDLM-2 and L540 cell lysates, respectively. Each immunprecipitate was incubated with His-tagged STAT3α protein in the absence or presence of MS-1020 at various concentrations. We found that both JAK2 and JAK3 immunoprecipitates efficiently phosphorylate His-tagged STAT3α protein in the absence of MS-1020. However, the addition of MS-1020 to the JAK3 kinase reactions effectively blocked His-tagged STAT3α tyrosine phosphorylation in a dose-dependent manner, whereas MS-1020 did not affect JAK2 kinase reactions (Fig 4A and B). These results suggest that MS-1020 binds directly with JAK3 and suppresses its catalytic activity. To examine the biochemical mechanism of action of MS-1020 on JAK3 inhibition, we investigated the effects of a 10-fold higher ATP concentration on JAK3 inhibition of this compound, and found that MS-1020 is an ATP-competitive JAK3 inhibitor (Fig 4B). Taken together, our data suggest that MS-1020 selectively inhibits JAK3 catalytic activity and subsequently leads to a block in STAT activation, and thus affects cell viability only in cancer cells with active JAK3/STAT signaling.
Fig 4.
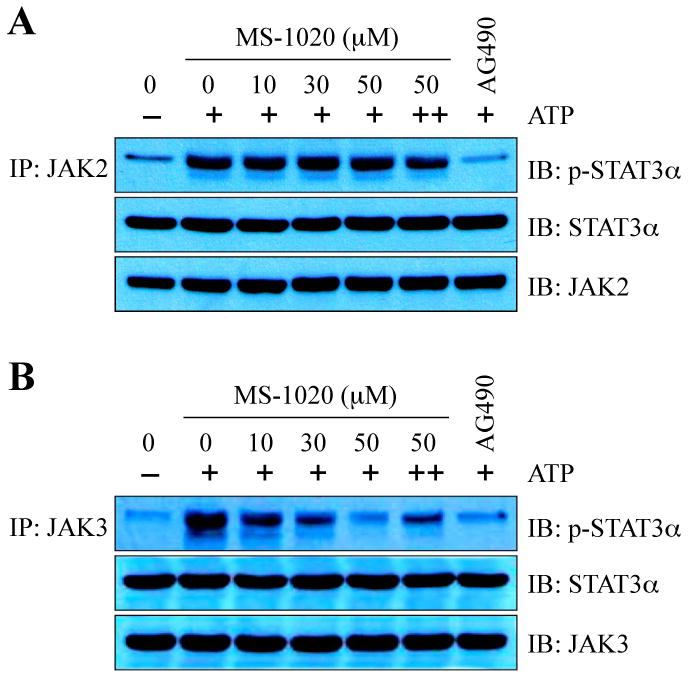
MS-1020 inhibits JAK3 kinase activity in vitro. JAK2 (A) or JAK3 (B) immunoprecipitates were used for in vitro kinase assay. Kinase reactions were performed by the addition of recombinant His-tagged STAT3α protein as a substrate, 2 μmol/L ATP (20 μmol/L ATP for competition experiments, ++), and MS-1020 at different concentrations or the JAK kinase inhibitor AG490 (150 μmol/L). The reaction products were resolved on SDS-PAGE and probed with antibodies specific for phospho-STAT3, STAT3, JAK2, and JAK3. Note that MS-1020 selectively blocks JAK3 kinase activity and that MS-1020 is an ATP-competitive JAK3 inhibitor. His-tagged STAT3α, JAK2, and JAK3 serve as a loading control.
MS-1020 down-regulates the expression of anti-apoptotic genes and induces apoptosis
Aberrant JAK/STAT signaling is thought to up-regulate the expression of anti-apoptotic genes and thus enable cancer cells to escape cancer treatment (Epling-Burnette et al, 2001; Mahboubi et al, 2001; Battle & Frank, 2002). To demonstrate that the decreased cell viability in MS-1020-treated L540 cells resulted from the induction of apoptosis, we first conducted a terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay. Cells were treated with vehicle (DMSO) alone or MS-1020 at various concentrations for 72 hours. As shown in Figure 5A, TUNEL-positive cells were increased ∼ 30-fold in 50 μM MS-1020-treated cells compared with controls (Fig 5A). The cleavage of Poly (ADP-ribose) polymerase (PARP) and cleaved caspase-3, both hallmarks of apoptosis, were substantially increased by MS-1020 in a dose-dependent manner (Fig 5B) (Decker et al, 2000).
Fig 5.
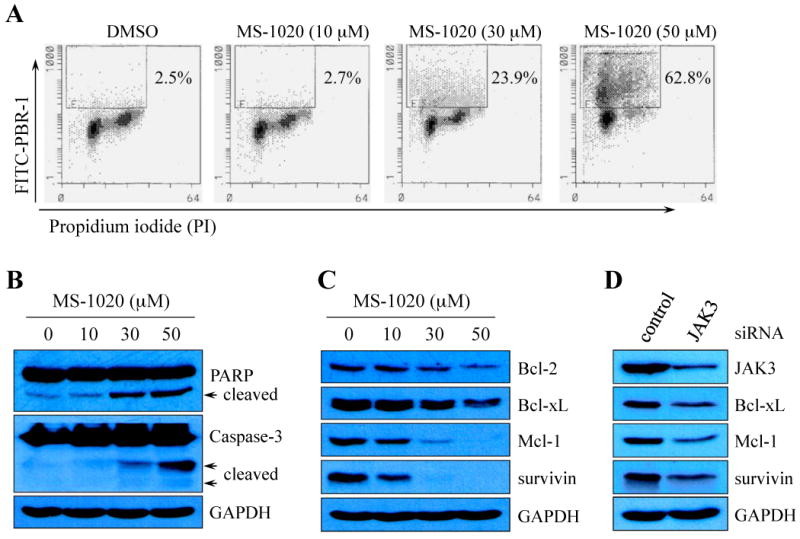
MS-1020 induces apoptosis by down-regulating the expression of anti-apoptotic genes. (A) A representative Flow Cytometry analysis for apoptosis. L540 cells were treated for 72 hours with vehicle (DMSO) alone or MS-1020 at various concentrations. Cells were harvested, stained with FITC-conjugated BrdU antibody and propidium iodide (PI), and subsequently subjected to Flow Cytometry. The percentages of TUNEL-positive cells are indicated. (B and C) L540 cells were treated with vehicle (DMSO) alone or MS-1020 at various concentrations for 48 hours. Whole-cell extracts were processed for Western blot analysis using antibodies specific for the molecules indicated. Treatment with MS-1020 increased cleaved fragments of Poly (ADP-ribose) polymerase (PARP) and caspase-3 (arrows in B), and decreased the expression of Bcl-2, Bcl-xL, Mcl-1, and survivin in a dose-dependent manner (C). (D) L540 cells were transiently transfected with either control siRNA or JAK3 siRNA. Total cell lysates were prepared 72 hours after transfection and processed for Western blot analysis. Note that treatment with JAK3 siRNA resulted in a reduction of anti-apoptotic gene expression. GAPDH serves as a loading control.
To a better understanding how MS-1020 affects apoptosis, we examined the effects of this reagent on the expression of anti-apoptotic genes, which are known STAT3 targets. L540 cells were treated with MS-1020 at various concentrations for 48 hours. Whole-cell extracts were processed for Western blot analysis using antibodies specific for Bcl-2, Bcl-xL, Mcl-1, and survivin. As expected, the expression of these anti-apoptotic genes was inhibited by treatment with MS-1020 in a dose-dependent manner (Fig 5C). These results suggest that MS-1020 decreases cancer cell survival by inducing programmed cell death via down-regulating the expression of anti-apoptotic genes.
Since MS-1020 inhibits JAK3/STAT signaling and induces apoptosis in L540 cells, we hypothesized that MS-1020-induced apoptosis resulted from JAK3 inhibition. To test this hypothesis, we treated L540 cells with either scrambled siRNA or JAK3 siRNA, and examined the effects of reduced JAK3 activity on anti-apoptotic gene expression. We detected the reduced expression of Bcl-xL, Mcl-1 and survivin, in JAK3 siRNA-treated L540 cells compared with controls (Fig 5D).
Discussion
The mammalian genomes encode multiple isoforms (4 JAKs and 7 STATs) of core JAK/STAT pathway components, many of which are co-expressed within the same cells and can form dimers to trigger a variety of specific effects in response to a diverse range of stimuli (Levy & Darnell, 2002). By contrast, the fruit fly Drosophila consists of only one JAK and one STAT, and thus can serve as an excellent model organism to identify the small molecule inhibitors of JAK/STAT signaling due to the low levels of redundancy within individual components of the JAK/STAT pathway (Arbouzva & Zeidler, 2006). Importantly, despite the simplicity of the Drosophila JAK/STAT pathway, molecular and functional analyses clearly indicate that the mode of action of the JAK/STAT pathway in Drosophila is similar to that of in mammals. (Hou et al, 2002; Bach et al, 2003; Arbouzva & Zeidler, 2006). To identify small molecule inhibitors of the JAK/STAT pathway, we performed a cell-based high throughput screen using a Drosophila cell line and identified Nb-(α-hydroxynaphthoyl)serotonin (MS-1020) as a JAK3/STAT signaling inhibitor. MS-1020 is a derivative of Nb-(p-feruloyl)serotonin, which was isolated from the extracts of Phragmites communis, Trin.. Interestingly, the “Reed” is one of the common aquatic plants and has long been used as the source of folk medicine to treat diseases, such as leukemia, breast cancer, rheumatoid arthritis, diabetes, and pulmonosis (Duke, 2000). In support of this, several conjugated serotonins have been identified from Carthamus tinctorius L. (Kawashima et al, 1998; Takii et al, 1999) and Amorphophallus konjac K. Koch. (Niwa et al, 2000), and have shown a variety of biological activities in the inhibition of pro-inflammatory cytokine production or tumor cell proliferation (Park & Schoene 2002; Roh et al, 2004; Kang et al, 2009).
JAKs are crucial in the signal transduction processes mediated by many cytokines, growth factors, and interleukins (Chilton & Hewetson, 2005; Schindler et al, 2007). JAK3 expression is preferentially expressed in leukocytes. By contrast, other JAK family members show relatively ubiquitous expression. Furthermore, JAK3 has been shown to mediate signals through the γc chain shared by for IL-2, IL-4, IL-7, IL-9, and IL-15 receptors in lymphoid cells, and the inhibition of JAK3 activity induces severe defects in T cell development and proliferation, suggesting the critical role of JAK3 in hematopoiesis (O'Shea et al, 2004; Pesu et al, 2005). Recent studies identified somatic mutations of JAK3 in a minority of acute megakaryoblastic leukemia (AMKL) patients and in AMKL-derived cell lines (Walters et al, 2006; Kiyoi et al, 2007; Malinge et al, 2008; Sato et al, 2008). Functional analysis of the JAK3 mutations showed that each of the mutations can transform Ba/F3 cells to factor-independent growth, indicating that these are JAK3 activating mutations. In particular, in vivo expression of JAK3A572V has been shown to cause a lethal hematopoietic malignancy with megakaryoblastic features, which include the infiltration of abnormally high numbers of megakaryocytes in the spleen and liver (Walters et al, 2006). Interestingly, an additional somatic mutation was recently reported in a high-risk childhood acute lymphoblastic leukemia case, even though functional consequence of this mutation remains undetermined (Mullighan et al, 2009). These observations, together with the restrictive lymphoid expression of JAK3, strongly suggest that JAK3 mutations contribute to the pathogenesis of hematopoietic malignancies and that inhibition of JAK3 is a logical target for therapeutic intervention in the malignancies with mutated JAK3 alleles. Several JAK3 inhibitors have indeed been identified in recent years, and the potential clinical use of these JAK3 inhibitors, including WHI-P154, PNU156804, NC1153 and CP-690550, in blood malignancies and immunosuppression has been implicated by numerous studies (Sudbeck et al, 1999; Stepkowski et al, 2002, Changelian et al, 2003; Stepkowski et al, 2005).
Botanical herbs have long been used as the source of folk medicine for the treatment of diseases, including leukemia, diabetes, and cardiovascular and liver disease (Eisenbrand et al, 2004; Grover & Yadav, 2004; Zhou et al, 2005; Stickel & Schuppan, 2007). These published observations clearly demonstrate the importance of natural products as a primary source for drug discovery and development targeting human diseases. The compound MS-1020 that we identified and characterized in this study is a synthetic compound derived from the extracts of Phragmites communis, Trin.. Our study showed for the first time that MS-1020 showed selective inhibition for JAK3 activity as compared with other JAK kinase members or with other oncogenic kinases, such as Src kinases, Akt, EGFR and ERK1/2 (Fig 2, Supplementary Fig 1). However, the specificity of MS-1020 for JAK3 still needs to be fully examined by evaluating the effects of this compound on a large panel of tyrosine and serine/threonine kinases in vitro. Nonetheless, MS-1020 was identified as an early lead compound that selectively inhibits JAK3/STAT signaling. Therefore, MS-1020 can be used as a starting point to develop a new class of drugs targeting aberrant JAK3/STAT signaling and can be used a pharmacological anti-cancer agent to target cancer cells harboring constitutively-active JAK3/STAT signaling.
Supplementary Material
Acknowledgments
This work was supported by the Children's Cancer Fund (Millwood, NY). We thank Dr. Charles Clevenger (Northwestern University) for providing Nb2 cells.
References
- Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- Bach EA, Vincent S, Zeidler MP, Perrimon N. A sensitized genetic screen to identify novel regulators and components of the Drosophila janus kinase/signal transducer and activator of transcription pathway. Genetics. 2003;165:1149–1166. doi: 10.1093/genetics/165.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg GH, Zhou R, Perrimon N. Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes & Development. 2005;19:1861–1870. doi: 10.1101/gad.1320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle TE, Frank DA. The role of STATs in apoptosis. Current Molecular Medicine. 2002;2:381–392. doi: 10.2174/1566524023362456. [DOI] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR, Cancer Genome Project Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, Rizzuti BJ, Sawyer PS, Perry BD, Brissette WH, McCurdy SP, Kudlacz EM, Conklyn MJ, Elliott EA, Koslov ER, Fisher MB, Strelevitz TJ, Yoon K, Whipple DA, Sun J, Munchhof MJ, Doty JL, Casavant JM, Blumenkopf TA, Hines M, Brown MF, Lillie BM, Subramanyam C, Shang-Poa C, Milici AJ, Beckius GE, Moyer JD, Su C, Woodworth TG, Gaweco AS, Beals CR, Littman BH, Fisher DA, Smith JF, Zagouras P, Magna HA, Saltarelli MJ, Johnson KS, Nelms LF, Des Etages SG, Hayes LS, Kawabata TT, Finco-Kent D, Baker DL, Larson M, Si MS, Paniagua R, Higgins J, Holm B, Reitz B, Zhou YJ, Morris RE, O'Shea JJ, Borie DC. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- Chilton BS, Hewetson A. Prolactin and growth hormone signaling. Current Topics in Developmental Biology. 2005;68:1–23. doi: 10.1016/S0070-2153(05)68001-5. [DOI] [PubMed] [Google Scholar]
- Darnell JE. Validating Stat3 in cancer therapy. Nature Medicine. 2005;11:595–596. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- Decker P, Isenberg D, Muller S. Inhibition of caspase-3-mediated poly(ADP-ribose) polymerase (PARP) apoptotic cleavage by human PARP autoantibodies and effect on cells undergoing apoptosis. The Journal of Biological Chemistry. 2000;275:9043–9046. doi: 10.1074/jbc.275.12.9043. [DOI] [PubMed] [Google Scholar]
- Diaz N, Minton S, Cox C, Bowman T, Gritsko T, Garcia R, Eweis I, Wloch M, Livingston S, Seijo E, Cantor A, Lee JH, Beam CA, Sullivan D, Jove R, Muro-Cacho CA. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clinical Cancer Research. 2006;12:20–28. doi: 10.1158/1078-0432.CCR-04-1749. [DOI] [PubMed] [Google Scholar]
- Duke JA. Handbook of Edible Weeds: Herbal Reference Library. CRC Press; 2000. pp. 144–145. [Google Scholar]
- Eisenbrand G, Hippe F, Jakobs S, Muehlbeyer S. Molecular mechanisms of indirubin and its derivatives: novel anticancer molecules with their origin in traditional Chinese phytomedicine. Journal of Cancer Research and Clinical Oncology. 2004;130:627–635. doi: 10.1007/s00432-004-0579-2. [DOI] [PubMed] [Google Scholar]
- Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang JM, Yang-Yen HF, Karras J, Jove R, Loughran TP., Jr Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. The Journal of Clinical Investigation. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flex E, Petrangeli V, Stella L, Chiaretti S, Hornakova T, Knoops L, Ariola C, Fodale V, Clappier E, Paoloni F, Martinelli S, Fragale A, Sanchez M, Tavolaro S, Messina M, Cazzaniga G, Camera A, Pizzolo G, Tornesello A, Vignetti M, Battistini A, Cavé H, Gelb BD, Renauld JC, Biondi A, Constantinescu SN, Foà R, Tartaglia M. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. The Journal of Experimental Medicine. 2008;205:751–758. doi: 10.1084/jem.20072182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover JK, Yadav SP. Pharmacological actions and potential uses of Momordica charantia: a review. Journal of Ethnopharmacology. 2004;93:123–132. doi: 10.1016/j.jep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Hou SX, Zheng Z, Chen X, Perrimon N. The Jak/STAT pathway in model organisms: emerging roles in cell movement. Developmental Cell. 2002;3:765–778. doi: 10.1016/s1534-5807(02)00376-3. [DOI] [PubMed] [Google Scholar]
- James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Natrure. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Jones AV, Chase A, Silver RT, Oscier D, Zoi K, Wang YL, Cario H, Pahl HL, Collins A, Reiter A, Grand F, Cross NC. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nature Genetics. 2009;41:446–449. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Park S, Kim YS, Lee S, Back K. Biosynthesis and biotechnological production of serotonin derivatives. Applied Microbiology and Biotechnology. 2009;83:27–34. doi: 10.1007/s00253-009-1956-1. [DOI] [PubMed] [Google Scholar]
- Kawashima S, Hayashi M, Takii T, Kimura H, Zhang HL, Nagatsu A, Sakakibara J, Murata K, Oomoto Y, Onozaki K. Serotonin derivative, N-(p-coumaroyl) serotonin, inhibits the production of TNF-alpha, IL-1alpha, IL-1beta, and IL-6 by endotoxin-stimulated human blood monocytes. Journal of Interferon & Cytokine Research. 1998;18:423–428. doi: 10.1089/jir.1998.18.423. [DOI] [PubMed] [Google Scholar]
- Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, Bass A, Marubayashi S, Heguy A, Garcia-Manero G, Kantarjian H, Offit K, Stone RM, Gilliland DG, Klein RJ, Levine RL. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nature Genetics. 2009;41:455–459. doi: 10.1038/ng.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BH, Yin CH, Guo Q, Bach EA, Lee H, Sandoval C, Jayabose S, Ulaczyk-Lesanko A, Hall DG, Baeg GH. A small-molecule compound identified through a cell-based screening inhibits JAK/STAT pathway signaling in human cancer cells. Molecular Cancer Therapeutics. 2008;7:2672–2680. doi: 10.1158/1535-7163.MCT-08-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirken RA, Rui H, Malabarba MG, Farrar WL. Identification of interleukin-2 receptor-associated tyrosine kinase p116 as novel leukocyte-specific Janus kinase. The Journal of Biological Chemistry. 1994;269:19136–19141. [PubMed] [Google Scholar]
- Kiyoi H, Yamaji S, Kojima S, Naoe T. JAK3 mutations occur in acute megakaryoblastic leukemia both in Down syndrome children and non-Down syndrome adults. Leukemia. 2007;21:574–576. doi: 10.1038/sj.leu.2404527. [DOI] [PubMed] [Google Scholar]
- Klampfer L. Signal transducers and activators of transcription (STATs): Novel targets of chemopreventive and chemotherapeutic drugs. Current Cancer Drug Targets. 2006;6:107–121. doi: 10.2174/156800906776056491. [DOI] [PubMed] [Google Scholar]
- Kota J, Caceres N, Constantinescu SN. Aberrant signal transduction pathways in myeloproliferative neoplasms. Leukemia. 2008;22:1828–1840. doi: 10.1038/leu.2008.236. [DOI] [PubMed] [Google Scholar]
- Levine RL, Loriaux M, Huntly BJ, Loh ML, Beran M, Stoffregen E, Berger R, Clark JJ, Willis SG, Nguyen KT, Flores NJ, Estey E, Gattermann N, Armstrong S, Look AT, Griffin JD, Bernard OA, Heinrich MC, Gilliland DG, Druker B, Deininger MW. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005;106:3377–3379. doi: 10.1182/blood-2005-05-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nature Review Molecular Cell Biology. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Levine RL. Janus kinase mutations. Seminars in Oncology. 2009;36:S6–11. doi: 10.1053/j.seminoncol.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Mahboubi K, Li F, Plescia J, Kirkiles-Smith NC, Mesri M, Du Y, Carroll JM, Elias JA, Altieri DC, Pober JS. Interleukin-11 up-regulates survivin expression in endothelial cells through a signal transducer and activator of transcription-3 pathway. Laboratory Investigation. 2001;81:327–334. doi: 10.1038/labinvest.3780241. [DOI] [PubMed] [Google Scholar]
- Malinge S, Ragu C, Della-Valle V, Pisani D, Constantinescu SN, Perez C, Villeval JL, Reinhardt D, Landman-Parker J, Michaux L, Dastugue N, Baruchel A, Vainchenker W, Bourquin JP, Penard-Lacronique V, Bernard OA. Activating mutations in human acute megakaryoblastic leukemia. Blood. 2008;112:4220–4226. doi: 10.1182/blood-2008-01-136366. [DOI] [PubMed] [Google Scholar]
- Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, Carver-Moore K, DuBois RN, Clark R, Aguet M, Schreiber RD. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Zhang J, Harvey RC, Collins-Underwood JR, Schulman BA, Phillips LA, Tasian SK, Loh ML, Su X, Liu W, Devidas M, Atlas SR, Chen IM, Clifford RJ, Gerhard DS, Carroll WL, Reaman GH, Smith M, Downing JR, Hunger SP, Willman CL. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson LM, Zhu J, Xie J, Malabarba MG, Sakamoto K, Wagner KU, Kirken RA, Rui H. Coactivation of janus tyrosine kinase (Jak)1 positively modulates prolactin-Jak2 signaling in breast cancer: recruitment of ERK and signal transducer and activator of transcription (Stat)3 and enhancement of Akt and Stat5a/b pathways. Molecular Endocrinology. 2007;21:2218–2232. doi: 10.1210/me.2007-0173. [DOI] [PubMed] [Google Scholar]
- Neubauer H, Cumano A, Müller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- Niwa T, Etoh H, Shimizu A, Shimizu Y. Cis-N-(p-Coumaroyl)serotonin from Konnyaku, Amorphophallus konjac K. Koch. Bioscience, Biotechnology, and Biochemistry. 2000;64:2269–2271. doi: 10.1271/bbb.64.2269. [DOI] [PubMed] [Google Scholar]
- Nosaka T, van Deursen JM, Tripp RA, Thierfelder WE, Witthuhn BA, McMickle AP, Doherty PC, Grosveld GC, Ihle JN. Defective lymphoid development in mice lacking Jak3. Science. 1995;270:800–802. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- Nybakken K, Vokes SA, Lin TY, McMahon AP, Perrimon N. A genome-wide RNA interference screen in Drosophila melanogater cells for new components of the Hh signaling pathway. Nature Genetics. 2005;37:1323–1332. doi: 10.1038/ng1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcaydu D, Harutyunyan A, Jäger R, Berg T, Gisslinger B, Pabinger I, Gisslinger H, Kralovics R. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nature Genetics. 2009;41:450–454. doi: 10.1038/ng.341. [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Pesu M, Borie DC, Changelian PS. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nature Reviews Drug Discovery. 2004;3:555–564. doi: 10.1038/nrd1441. [DOI] [PubMed] [Google Scholar]
- Park SY, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, Miyake K, Nakauchi H, Shirasawa T, Saito T. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–782. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- Park JB, Schoene N. Synthesis and characterization of N-coumaroyltyramine as a potent phytochemical which arrests human transformed cells via inhibiting protein tyrosine kinases. Biochemical and Biophysical Research Communications. 2002;292:1104–1110. doi: 10.1006/bbrc.2002.6752. [DOI] [PubMed] [Google Scholar]
- Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- Pesu M, Candotti F, Husa M, Hofmann SR, Notarangelo LD, O'Shea JJ. Jak3, severe combined immunodeficiency, and a new class of immunosuppressive drugs. Immunological Reviews. 2005;203:127–142. doi: 10.1111/j.0105-2896.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- Pesu M, Laurence A, Kishore N, Zwillich SH, Chan G, O'Shea JJ. Therapeutic targeting of Janus kinases. Immunological Reviews. 2008;223:132–142. doi: 10.1111/j.1600-065X.2008.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiterer G, Yen A. Inhibition of the janus kinase family increases extracellular signal-regulated kinase 1/2 phosphorylation and causes endoreduplication. Cancer Research. 2006;66:9083–9089. doi: 10.1158/0008-5472.CAN-06-0972. [DOI] [PubMed] [Google Scholar]
- Roh JS, Han JY, Kim JH, Hwang JK. Inhibitory effects of active compounds isolated from safflower (Carthamus tinctorius L.) seeds for melanogenesis. Biological & Pharmaceutical Bulletin. 2004;27:1976–1978. doi: 10.1248/bpb.27.1976. [DOI] [PubMed] [Google Scholar]
- Rui H, Kirken RA, Farrar WL. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. The Journal of Biological Chemistry. 1994;269:5364–5368. [PubMed] [Google Scholar]
- Sato T, Toki T, Kanezaki R, Xu G, Terui K, Kanegane H, Miura M, Adachi S, Migita M, Morinaga S, Nakano T, Endo M, Kojima S, Kiyoi H, Mano H, Ito E. Functional analysis of JAK3 mutations in transient myeloproliferative disorder and acute megakaryoblastic leukaemia accompanying Down syndrome. British Journal of Haematology. 2008;141:681–688. doi: 10.1111/j.1365-2141.2008.07081.x. [DOI] [PubMed] [Google Scholar]
- Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. The Journal of Biological Chemistry. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- Schindler C, Shuai K, Prezioso VR, Darnell JE., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992;257:809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Shuai K, Horvath CM, Huang LH, Qureshi SA, Cowburn D, Darnell JE., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994;76:821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Shuai K, Schindler C, Prezioso VR, Darnell JE., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992;285:1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Shuai K, Stark GR, Kerr IM, Darnell JE., Jr A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science. 1993a;261:1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- Shuai K, Ziemiecki A, Wilks AF, Harpur AG, Sadowski HB, Gilman MZ, Darnell JE., Jr Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993b;366:580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- Stepkowski SM, Erwin-Cohen RA, Behbod F, Wang ME, Qu X, Tejpal N, Nagy ZS, Kahan BD, Kirken RA. Selective inhibitor of Janus tyrosine kinase 3, PNU156804, prolongs allograft survival and acts synergistically with cyclosporine but additively with rapamycin. Blood. 2002;99:680–689. doi: 10.1182/blood.v99.2.680. [DOI] [PubMed] [Google Scholar]
- Stepkowski SM, Kao J, Wang ME, Tejpal N, Podder H, Furian L, Dimmock J, Jha A, Das U, Kahan BD, Kirken RA. The Mannich base NC1153 promotes long-term allograft survival and spares the recipient from multiple toxicities. Journal of Immunology. 2005;175:4236–4246. doi: 10.4049/jimmunol.175.7.4236. [DOI] [PubMed] [Google Scholar]
- Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Digestive and Liver Disease. 2007;39:293–304. doi: 10.1016/j.dld.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Sudbeck EA, Liu XP, Narla RK, Mahajan S, Ghosh S, Mao C, Uckun FM. Structure-based design of specific inhibitors of Janus kinase 3 as apoptosis-inducing antileukemic agents. Clinical Cancer Research. 1999;5:1569–1582. [PubMed] [Google Scholar]
- Takii T, Hayashi M, Hiroma H, Chiba T, Kawashima S, Zhang HL, Nagatsu A, Sakakibara J, Onozaki K. Serotonin derivative, N-(p-Coumaroyl)serotonin, isolated from safflower (Carthamus tinctorius L.) oil cake augments the proliferation of normal human and mouse fibroblasts in synergy with basic fibroblast growth factor (bFGF) or epidermal growth factor (EGF) Journal of Biochemistry. 1999;125:910–915. doi: 10.1093/oxfordjournals.jbchem.a022368. [DOI] [PubMed] [Google Scholar]
- Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70:313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Walters DK, Mercher T, Gu TL, O'Hare T, Tyner JW, Loriaux M, Goss VL, Lee KA, Eide CA, Wong MJ, Stoffregen EP, McGreevey L, Nardone J, Moore SA, Crispino J, Boggon TJ, Heinrich MC, Deininger MW, Polakiewicz RD, Gilliland DG, Druker BJ. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell. 2006;10:65–75. doi: 10.1016/j.ccr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Watling D, Guschin D, Müller M, Silvennoinen O, Witthuhn BA, Quelle FW, Rogers NC, Schindler C, Stark GR, Ihle JN, Kerr IM. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993;366:166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nature reviews Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- Zeidler MP, Bach EA, Perrimon N. The roles of the Drosophila JAK/STAT pathway. Oncogene. 2000;19:2598–2606. doi: 10.1038/sj.onc.1203482. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. Journal of Clinical Pharmacology. 2005;45:1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.