Fig. 5. HRI eIF2α kinase.
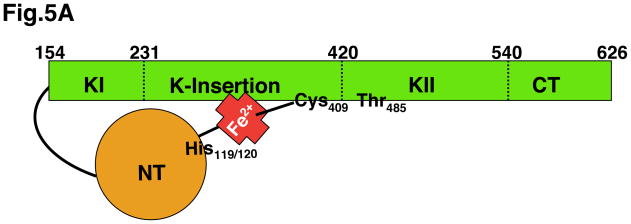
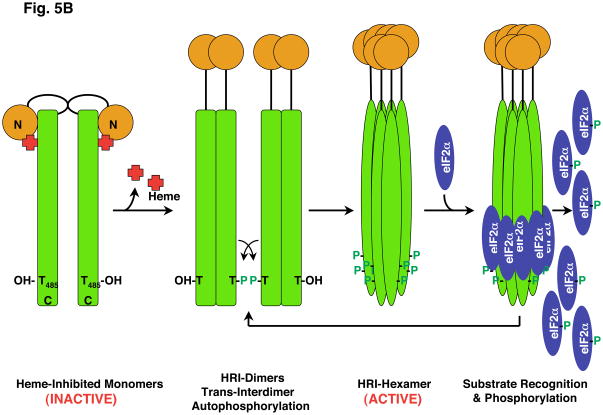
A. An inactive, heme-bound HRI 76 kDa subunit is schematically shown. Heme tethers the regulatory N-terminal (NT) subunit via His119/His120 to Cys409 in the Cys-Pro HRM in the kinase insert (KI) domain. Mutation of either of these His residues results in “ligand switching” to the other (Igarashi et al., 2008). This large insert (spanning residues 231–420) in the kinase domain is a hallmark of all eIF2α kinases. The HRI protein undergoes phosphorylation at multiple sites, but the Thr residue corresponding to Thr485 in the HRI kinase K-II activation loop is conserved in all eIF2α kinases and incurs autophosphorylation. B. A plausible model for HRI activation and subsequent eIF2α phosphorylation is illustrated, based on that reported for PKR eIF2α kinase (Dar et al., 2005; Dey et al., 2005) and various reports in the HRI literature (Bauer et al. 2001; Miksanova et al., 2006; Igarashi et al., 2008). The HRI NT domain is essential for HRI-homodimerization/oligomerization. Heme depletion results in the loss of the HRI NT-heme tethering to the KI domain, resulting in homodimerization, trans-interdimer autophosphorylation, conformational changes leading to the active HRI homotrimeric/hexameric structure, eIF2α recognition and phosphorylation at Ser51.