Abstract
Background:
While Parkinson disease (PD) is consistently associated with impaired olfaction, one study reported better olfaction among Parkin mutation carriers than noncarriers. Whether olfaction differs between Parkin mutation heterozygotes and carriers of 2 Parkin mutations (compound heterozygotes) is unknown.
Objective:
To assess the relationship between Parkin genotype and olfaction in PD probands and their unaffected relatives.
Methods:
We administered the University of Pennsylvania Smell Identification Test (UPSIT) to 44 probands in the Consortium on Risk for Early-Onset Parkinson Disease study with PD onset ≤50 years (10 Parkin mutation heterozygotes, 9 compound heterozygotes, 25 noncarriers) and 80 of their family members (18 heterozygotes, 2 compound heterozygotes, 60 noncarriers). In the probands, linear regression was used to assess the association between UPSIT score (outcome) and Parkin genotype (predictor), adjusting for covariates. Among family members without PD, we compared UPSIT performance in heterozygotes vs noncarriers using generalized estimating equations, adjusting for family membership, age, gender, and smoking.
Results:
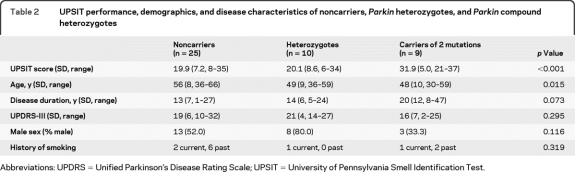
Among probands with PD, compound heterozygotes had higher UPSIT scores (31.9) than heterozygotes (20.1) or noncarriers (19.9) (p < 0.001). These differences persisted after adjustment for age, gender, disease duration, and smoking. Among relatives without PD, UPSIT performance was similar in heterozygotes (32.5) vs noncarriers (32.4), and better than in heterozygotes with PD (p = 0.001).
Conclusion:
Olfaction is significantly reduced among Parkin mutation heterozygotes with PD but not among their heterozygous relatives without PD. Compound heterozygotes with PD have olfaction within the normal range. Further research is required to assess whether these findings reflect different neuropathology in Parkin mutation heterozygotes and compound heterozygotes.
Mutations in the Parkin gene (PARK2; OMIM 600116)1,2 are the most common genetic risk factors for early-onset Parkinson disease (PD) (EOPD)3–13; however, the role of heterozygous Parkin mutations in the pathogenesis of PD remains controversial.14–17 While a comprehensive study showed a similar frequency of heterozygous point mutations in PD cases and controls,16 PET studies show reduced fluorodopa uptake in nigrostriatal terminals in the caudate and posterior putamen of both symptomatic and asymptomatic Parkin heterozygotes compared to controls, similar to the reduction found in sporadic PD.18–20
Impairment in olfactory function is one of the earliest manifestations of idiopathic PD and has been reported up to 4 years before motor manifestations.21 While impaired olfaction is frequently associated with PD, olfactory identification was reported to be better among 22 patients with parkinsonism with one or more Parkin mutations when compared to noncarrier patients with PD.22,23 Four of these carriers of Parkin mutations were heterozygotes (Dr. N. Khan, personal communication). Olfactory performance in Parkin mutation heterozygotes vs compound heterozygotes has never been reported. The purpose of this study was to compare olfactory function among carriers of a single Parkin mutation, carriers of 2 Parkin mutations, and noncarriers with and without PD.
METHODS
Participants.
This study included 44 individuals with EOPD (probands) who participated in Part II of the Consortium on Risk for Early-Onset Parkinson Disease study (CORE-PD), and 80 of their family members. The details of the CORE-PD study are described elsewhere.24 In brief, patients with PD were recruited from 13 sites based on age at onset (AAO) of PD ≤50 years and performance on the Mini-Mental State Examination (MMSE)25 >23 to ensure that a reliable history could be obtained from each subject. A blood sample for DNA was sent to the NINDS Human Genetics Resource Center DNA and Cell Line Repository (http://ccr.coriell.org). In part II of CORE-PD, patients who carried Parkin mutations and a sample of those who did not carry Parkin mutations, as well as their family members, underwent a detailed neurologic, neuropsychological, and psychiatric assessment. Beginning in 2007, olfactory function was measured by the University of Pennsylvania Smell Identification Test (UPSIT,26 Sensonics, Inc., Haddonfield, NJ). The UPSIT consists of 40 standardized encapsulated odors. Raw scores are calculated as the simple number of correct identifications ranging from 0 to 40; higher scores indicate better olfaction. Anosmia is defined as a score of 18 or lower and severe microsmia is defined as a score of 19–25 in people above age 15.27 The reliability of UPSIT is well-established.26 The UPSIT was obtained only if participants denied any upper respiratory illness, as suggested by the UPSIT manual. Smoking history was obtained on all participants. All examiners were unaware of the genetic status of the participants at the time of recruitment and thereafter.
Probands and family members were screened for Parkin mutations, as well as for common mutations in LRRK2 and glucocerebrosidase (GBA), using previously described methods,24 after the examination was complete. In addition, most participants, including 31 of the 43 probands with PD and all family members, were screened for mutations in α-synuclein (SNCA; A157T, A88P, and E136K), DJ-1 (L166P, M26I, D149A, and A104T), and PTEN-induced putative kinase 1 (PINK-1; W437X and G309D).28 None were aware of their mutation status. Carriers of LRRK2 or GBA were excluded from the analyses. No carriers of mutations in SNCA, DJ-1, or PINK-1 were detected.
Standard protocol approvals, registrations, and patient consents.
Institutional review boards at all participating sites approved the protocols and consent procedures. Written informed consent was obtained from all participants in the study.
Data analysis.
Probands.
Demographics, clinical characteristics, and UPSIT performance were compared among the 3 groups defined by Parkin genotype using univariate analysis of variance and χ2 tests as appropriate. A linear regression model was constructed to assess the association between UPSIT score (outcome) and mutation status (predictor), adjusting for age, gender, disease duration, and history of smoking (past and current vs never). Finally, we compared the proportion of probands with severe olfactory impairment (either severe microsmia or anosmia), defined as a score of 25 or lower (UPSIT manual)27 among the genetic groups.
Unaffected family members.
Given that only 2 family members without PD carried 2 Parkin mutations (both compound heterozygotes), we restricted the analysis of UPSIT performance in family members to Parkin mutation heterozygotes and noncarriers. We used generalized estimating equations (GEE) to adjust for familial clustering, age, gender, and history of smoking. Finally, we compared UPSIT performance between Parkin mutation heterozygotes with and without PD using the GEE model, to assess whether hyposmia was associated with PD among mutation carriers.
RESULTS
Probands with PD.
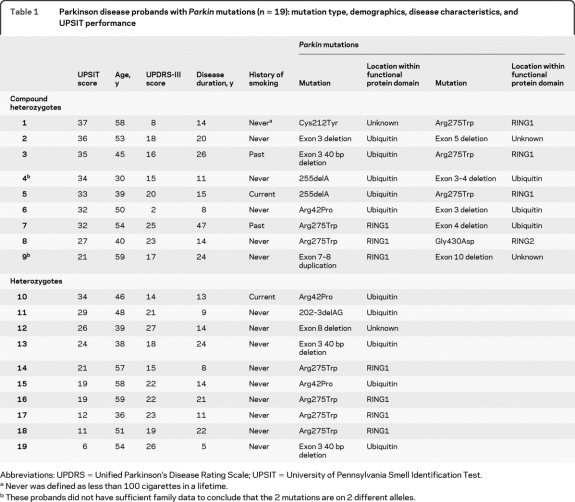
Probands with PD included 9 carriers of 2 Parkin mutations (all compound heterozygotes), 10 Parkin mutation heterozygotes, and 25 noncarriers. The specific mutations in Parkin carriers are described in table 1. All the Parkin mutations reported are considered pathogenic.24 Seven of the 9 carriers of 2 Parkin mutations had sufficient family data to conclude that the 2 Parkin mutations are on 2 different alleles (table 1).
Table 1.
Parkinson disease probands with Parkin mutations (n = 19): mutation type, demographics, disease characteristics, and UPSIT performance
Abbreviations: UPDRS = Unified Parkinson's Disease Rating Scale; UPSIT = University of Pennsylvania Smell Identification Test.
Never was defined as less than 100 cigarettes in a lifetime.
These probands did not have sufficient family data to conclude that the 2 mutations are on 2 different alleles.
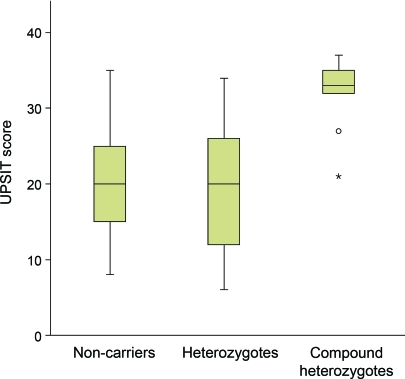
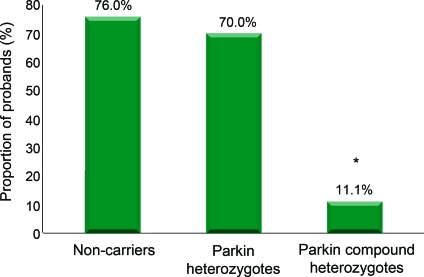
Probands with PD who carried 2 Parkin mutations performed significantly better on the UPSIT (31.9) than probands who were heterozygotes (20.1) or noncarriers (19.9), despite longer disease duration (figure 1, table 2). Mean UPSIT performance of probands who were Parkin mutation heterozygotes was within the range of severe microsmia (19–25), and was not significantly different from probands who were noncarriers. In a linear regression model including all probands with PD, carrying 2 Parkin mutations was associated with higher UPSIT scores when compared to Parkin mutation heterozygotes (10.9-point difference, p = 0.005) or to noncarriers (10.7-point difference, p = 0.003), after adjustment for gender, age, disease duration, and history of smoking. We did not find any association between olfaction and Parkin mutation type (point mutations vs gene dosage alterations), or with disease severity indicators, including Unified Parkinson's Disease Rating Scale–III and disease duration. The proportion of Parkin mutation heterozygote and noncarrier probands who were either severely microsmic or anosmic combined (i.e., UPSIT score lower than 26) was higher than Parkin compound heterozygotes (p = 0.02, figure 2). The significant difference between Parkin mutation heterozygotes and compound heterozygotes did not change after excluding the 2 compound heterozygotes on whom there were insufficient family data to confirm compound heterozygocity status.
Figure 1. Box plot showing the University of Pennsylvania Smell Identification Test (UPSIT) scores in early-onset Parkinson disease participants who are Parkin compound heterozygotes, heterozygotes, or noncarriers.
Table 2.
UPSIT performance, demographics, and disease characteristics of noncarriers, Parkin heterozygotes, and Parkin compound heterozygotes
Abbreviations: UPDRS = Unified Parkinson's Disease Rating Scale; UPSIT = University of Pennsylvania Smell Identification Test.
Figure 2. Proportion of probands with either anosmia or severe microsmia (University of Pennsylvania Smell Identification Test <26).
Family members without PD.
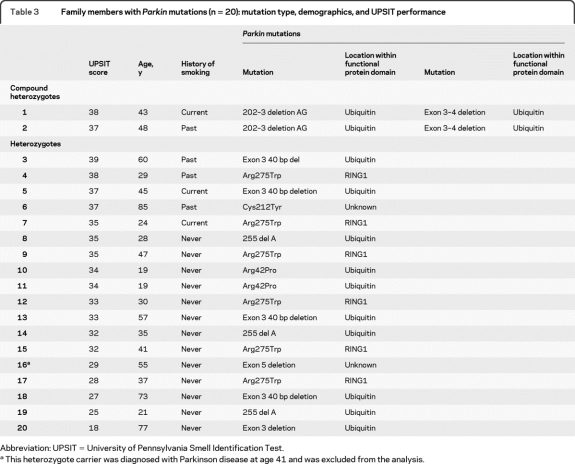
Family members included 80 individuals from 37 families: 60 noncarriers, 18 Parkin mutation heterozygotes, and 2 compound heterozygotes. The specific mutations in family members who were Parkin carriers are described in table 3. A single heterozygote family member was diagnosed with PD and was excluded from the analysis (table 3). Among 79 family members without PD, the performance on the UPSIT of Parkin mutation heterozygotes (mean 32.5, range 18–39, table 3) was similar to that of noncarriers (mean 32.4, range 17–39, p = 0.69, adjusted for familial clustering, age, gender, and history of smoking in GEE model).
Table 3.
Family members with Parkin mutations (n = 20): mutation type, demographics, and UPSIT performance
Abbreviation: UPSIT = University of Pennsylvania Smell Identification Test.
This heterozygote carrier was diagnosed with Parkinson disease at age 41 and was excluded from the analysis.
Among individuals who were heterozygous carriers of Parkin mutations (probands with PD and relatives without PD), performance on the UPSIT was better in relatives without PD than in probands with EOPD (32.5 vs 20.1, p = 0.001, adjusted for familial clustering, age, gender, and history of smoking in a GEE model); however, in 12% (2/17) of the heterozygote family members without PD (from 2 different families) UPSIT performance was consistent with severe microsmia or anosmia. Both carried deletions in the Parkin gene (table 3). Both Parkin compound heterozygotes without PD had normal smell performance (table 3); however, statistical analysis was not performed given the small number (n = 2).
DISCUSSION
Impairment in olfactory function is an early finding in idiopathic PD, and is not associated with severity of motor function or medication dosage.29,30 Our study of olfaction in a genotyped sample of EOPD confirms previous reports of better olfaction in carriers of 2 Parkin mutations with PD,22,23 and demonstrates impaired olfaction in Parkin mutation heterozygotes with PD, similar to that of people with PD who were Parkin noncarriers and significantly worse than compound heterozygotes with PD. The role of Parkin mutation heterozygosity in the pathogenesis of PD is controversial.14–17 Data supporting the hypothesis that heterozygous mutations convey a risk for PD include imaging findings18–20 and studies showing an increased frequency of heterozygous carriers in PD cases compared to controls.15 Our finding of similar olfactory performance in Parkin heterozygotes with PD and noncarriers with PD may be viewed as consistent with the hypothesis that Parkin mutation heterozygosity is not an independent risk factor for PD. Alternatively, poor performance on the UPSIT may reflect a different distribution of pathology among Parkin mutation heterozygotes compared to compound heterozygotes.
Olfactory impairment in PD is associated with Lewy body infiltration of the olfactory bulb and tract.31 Neuropathologic staging of PD suggests the presence of Lewy bodies in the olfactory bulb in Braak stages 1–2, even before the disease reaches the substantia nigra (stage 3).32 To our knowledge, 7 autopsies of individuals with Parkin mutations have been reported. Only 2 of the 6 homozygotes/compound heterozygotes had Lewy bodies,33,34 whereas the clinical course and pathology from a single autopsy of a Parkin mutation heterozygote was consistent with progressive supranuclear palsy.35 Based on our findings of different olfactory performance in Parkin mutation heterozygotes and Parkin compound heterozygotes, we hypothesize that better UPSIT performance is inversely correlated with Lewy body pathology, and that Parkin heterozygotes with PD have Lewy bodies in the olfactory bulb; however, autopsy data are lacking, and further research is required to address the relationship between olfactory performance and the underlying disease mechanism.
Limitations of our study include its sample size, which did not allow us to analyze point mutation carriers and gene dosage alterations carriers in the Parkin gene separately. While we excluded carriers of common mutations in LRRK2 and GBA to avoid confounders, we only screened for specific mutations and did not sequence the α-synuclein DJ-1 or PINK-1 genes, which are associated with impaired olfaction.36
When Parkin mutation heterozygotes with and without PD were compared, olfactory impairment was associated with PD, supporting the notion that hyposmia in Parkin mutation heterozygotes is related to PD rather than to Parkin genotype. However, we also noted that 2 (12%) of the Parkin mutation heterozygotes without PD manifested impaired olfaction. In light of 2 prospective studies that demonstrated that 10%–13% of individuals who had both hyposmia and abnormal functional imaging at baseline developed PD over a 2- to 5-year period,37,38 a longitudinal follow-up of these carriers is warranted to determine whether hyposmia can be used as an early marker of PD in Parkin heterozygous mutation carriers, and whether Parkin mutation heterozygosity is indeed a risk factor for PD.
ACKNOWLEDGMENT
The authors thank Dr. Paul Greene for his assistance in recruitment and manuscript review and Dr. Howard Andrews for database support.
- AAO
- age at onset
- CORE-PD
- Consortium on Risk for Early-Onset Parkinson Disease study
- EOPD
- early-onset Parkinson disease
- GEE
- generalized estimating equation
- MMSE
- Mini-Mental State Examination
- PD
- Parkinson disease
- UPSIT
- University of Pennsylvania Smell Identification Test
Editorial, page 312
AUTHOR AFFILIATIONS
From the Department of Neurology (R.N.A., R.O., E.C., H.M.-S., M.-X.T., L.R., E.L., R.D., C.W., S. Fahn, S. Frucht, B.F., R.M.O., K.M.), Taub Institute for Research on Alzheimer's Disease and the Aging Brain (R.N.A., R.O., M.-X.T., E.L., B.R., M.V., S.K., L.N.C., K.M.), Gertrude H. Sergievsky Center (R.O., E.L., L.C.), Department of Pathology and Cell Biology (L.N.C.), and Center for Human Genetics (L.N.C.), College of Physicians and Surgeons, and Department of Epidemiology (R.O., E.L.), Mailman School of Public Health, Columbia University, New York, NY; Department of Neurology (A.S., A.C.), University of Pennsylvania Health System, Philadelphia; Division of Epidemiology (R.O., K.M.), New York State Psychiatric Institute, New York; Department of Neurology/Movement Disorder Section (C.C.), Rush University, Chicago, IL; The Institute for Neurodegenerative Disorders (D.J.), New Haven, CT; Struthers Parkinson's Center (M.N.), Park Nicollet Clinic, Golden Valley, MN; The Alan and Barbara Mirken Department of Neurology (S.B.), Beth Israel Medical Center, New York, NY; Department of Neurology (S.B.), Albert Einstein College of Medicine, Bronx, NY; Dr. John T. Macdonald Foundation (W.K.S.), Department of Human Genetics, John P. Hussman Institute for Human Genomics, Miller School of Medicine, University of Miami, Miami, FL; Parkinson's Institute (C.T.), Sunnyvale, CA; Marshfield Clinic (S.M.), Department of Neurology, Marshfield, WI; Department of Neurology (M.R., K.E.N.), NorthShore University HealthSystem, Evanston, IL; Department of Neurology (M.R., K.E.N.), The University of Chicago, Pritzker School of Medicine, Chicago, IL; Parkinson's Disease and Movement Disorders Center of NeuroHealth (J.H.F.), Warwick, RI; Department of Clinical Neurosciences (J.H.F.), The Warren Alpert School of Medicine of Brown University, Providence, RI; Department of Neurology (R.P.), College of Medicine, University of Tennessee Health Science Center, Memphis; Morris K. Udall Parkinson's Disease Research Center of Excellence (L.M.), Department of Psychiatry and Behavioral Sciences (L.M.), and Department of Neurology and Neurological Sciences (L.M.), Johns Hopkins University School of Medicine, Baltimore, MD; Medical College of Wisconsin (B.H.), Milwaukee; and Department of Psychiatry (K.M.), Columbia University Medical Center, New York, NY.
STUDY FUNDING
Supported by NIH NS36630, UL1 RR024156 (K.S.M.), NS050487, NS060113 (L.N.C.), the Parkinson's Disease Foundation (K.S.M., S.F., and L.N.C.), and P50 NS039764 (W.K.S.). R.N.A. was supported by a Parkinson's Disease Foundation H. Houston Merritt Fellowship in Movement Disorders.
DISCLOSURE
Dr. Alcalay has received publishing royalties for Early Onset Parkinson's Disease (Cyberounds, 2010) and receives research support from the Brookdale Foundation. Dr. Siderowf serves on scientific advisory boards for Teva Pharmaceutical Industries Ltd., NeuroSearch, and the Michael J. Fox Foundation; has received speaker honoraria from Teva Pharmaceutical Industries Ltd.; serves as a consultant for Merck Serono, Schering-Plough Corp., Teva Pharmaceutical Industries Ltd., Supernus Pharmaceuticals, Inc.; and has served as a consultant on manganese litigation. Dr. Ottman serves on the scientific advisory board for and holds stock options in Trigeminal Solutions, Inc; has received funding for travel from the International League Against Epilepsy, Fondazione Ettore Majorana E Centro, National Epifellows Forum, the National Institute for Mental Health, and Coriell Institute for Medical Research; serves as a consultant to Ortho-McNeil Janssen Scientific Affairs, LLC.; and has received research support from the NIH. Dr. Caccappolo, Ms. Mejia-Santana, Dr. Tang, and Dr. Rosado report no disclosures. Dr. Louis has served on a scientific advisory board for Pfizer Inc; and has received research support from the NIH (NINDS, NIA) and the Parkinson's Disease Foundation. Ms. Ruiz reports no disclosures. Dr. Waters receives publishing royalties for Diagnosis and Management of Parkinson's Disease (Oxford University Press, 2009); serves as a consultant for Teva Pharmaceutical Industries Ltd.; serves on speakers' bureaus for Teva Pharmaceutical Industries Ltd. and Boehringer Ingelheim; and receives research support from Solvay Pharmaceuticals, Inc., UCB, and Novartis. Dr. Fahn serves on scientific advisory boards for Intech Pharma Pvt. Ltd., IMPAX Laboratories, Inc., Boehringer Ingelheim, Vernalis plc Merz Pharmaceuticals, LLC, Oxford BioMedica Plc, GE Healthcare, RJG Foundation, and Lundbeck, Inc.; has received funding for travel from Boerhinger Ingelheim and Sun Pharmaceutical Industries Ltd.; serves on the editorial board of Current Neurology and Neurosurgery Report; receives publishing royalties for Principles and Practice of Movement Disorders (Elsevier, 2007); has served as a consultant in medico-legal cases; and receives research support from the Parkinson's Disease Foundation and the Smart Family Foundation. Dr. Cote has serves as a consultant for Teva Pharmaceutical Industries Ltd. Dr. Frucht has received funding for travel from Jazz Pharmaceuticals, Lundbeck Inc., and Merz Pharmaceuticals, LLC; receives publishing royalties for Movement Disorders Emergencies (Humana Press, 2005); and has served as a consultant for UCB, Jazz Pharmaceuticals, Merz Pharmaceuticals, LLC, Lundbeck, Inc., GE Healthcare, and Allergan, Inc. Dr. Ford serves on a scientific advisory board for Medtronic, Inc. Dr. Orbe-Reilly, Ms. Ross, Dr. Verbitsky, and Mr. Kisselev report no disclosures. Dr. Comella serves on the editorial board of Sleep Medicine; receives publishing royalties from UpToDate; and serves as a consultant for Allergan, Inc., Ipsen, Eisai Inc., Merz Pharmaceuticals, LLC, and UCB. Dr. Colcher has received speaker honoraria from the Robert Wood Johnson, Plan 365, Healthlogix, and Advanced health Media; and serves on speakers' bureaus for Lundbeck, Inc., Teva Pharmaceutical Industries Ltd., and Ipsen. Dr. Jennings serves on a scientific advisory board for Genzyme Corporation and serves on the speakers' bureaus of Lundbeck Inc. and Teva Pharmaceutical Industries Ltd. Dr. Nance serves on scientific advisory boards for the Spastic Paraplegia Foundation and Parkinson Study Group; receives publishing royalties for Juvenile Huntington's Disease and Other Trinucleotide Repeat Disorders (Oxford University Press, 2009); receives research support from Schwarz Biosciences Inc., NeuroSearch, IMPAX Laboratories, Inc., Medivation, Inc., Neuraltus Pharmaceuticals, Inc., Teva Pharmaceutical Industries Ltd., Juvantia Pharma Ltd., the NIH (NINDS, NHGRI, NCCAM), the National Parkinson Foundation, the Huntington Disease Society of America, the Michael J. Fox Foundation, and Northwestern Dixon Foundation; and her spouse serves on speakers' bureaus for Genentech, Inc. and Schering-Plough Corp. Dr. Bressman serves on scientific advisory boards for the Bachmann-Strauss Dystonia & Parkinson Foundation and the Michael J. Fox Foundation; receives royalties from publication of Clinical Diagnosis and Management of Dystonia (Informa UK Ltd, 2007); and receives research support from the NIH/NINDS, the Michael J. Fox Foundation, and the Bachmann-Strauss Dystonia & Parkinson Foundation. Dr. Scott is co-inventor of a patent re: use of genetic data for risk assessment in age-related macular degeneration, licensed by ArcticDx. Dr. Tanner has served on scientific advisory boards for Allergan, Inc., the Michael J. Fox Foundation, and the Spasmodic Dysphonia Association; has served as a consultant for Stanford University, Pacific Health Research Institute, Sun Health Research Institute, IMPAX Laboratories, Inc., Lundbeck, Inc., and Solstice Neurosciences, Inc.; and has received research support from the Welding Products Manufacturer's Group, the NIH (NINDS, NIEHS), DOD, AHRQ, Parkinson's Institute, Parkinson's Disease Foundation, Michael J. Fox Foundation, Brin Foundation, Stanford University/John Blume Foundation, and Parkinson Alliance (Unity Walk). Dr. Mickel reports no disclosures. Dr. Rezak serves on speakers' bureaus for and has received speaker honoraria from Teva Pharmaceutical Industries Ltd., Allergan Inc., Medtronic, Inc., Novartis, and GlaxoSmithKline; and serves as a consultant for Teva Pharmaceutical Industries Ltd. Dr. Novak receives research support from Cyberonics, Inc., GE Healthcare, the NIH, and the Parkinson's Disease Research Society. Dr. Friedman serves on scientific advisory boards or as a consultant for Teva Pharmaceutical Industries Ltd., EMD Serono, Inc., Biogen Idec, and ACADIA Pharmaceuticals; has received speaker honoraria from Teva Pharmaceutical Industries Ltd., Boehringer Ingelheim, GlaxoSmithKline, United Biosource Corporation, and AstraZeneca; serves as Editor-in-Chief of Medicine & Health/Rhode Island and on the editorial boards of Parkinsonism & Related Disorders and Neurology Reviews; receives publishing royalties for Making the Connection between Brain and Behavior: Coping with Parkinson's Disease (Demos Health, 2007); serves on speakers' bureaus for Teva Pharmaceutical Industries Ltd., Boehringer Ingelheim, GlaxoSmithKline; and receives research support from Teva Pharmaceutical Industries Ltd., Boehringer Ingelheim, GlaxoSmithKline, Pfizer Inc, Cephalon, Inc., ACADIA Pharmaceuticals, EpiVax, Inc., Valeant Pharmaceuticals International, the NIH, and the Michael J. Fox Foundation. Dr. Pfeiffer serves on a scientific advisory board for the National Parkinson Foundation; serves on the editorial board of Parkinsonism and Related Disorders; receives publishing royalties for Parkinson's Disease (Taylor & Francis, 2008), Parkinson's Disease and Nonmotor Dysfunction (Humana, 2008), and Neuro-Gastroenterology (Butterworth-Heinemann, 2008); serves as a consultant for Solvay Pharmaceuticals, Inc., Theravance, Inc., Genactis, Inc., and Schlesinger Associates; serves on speakers' bureaus for and has received speaker honoraria from Boehringer Ingelheim, Novartis, and Teva Pharmaceutical Industries Ltd.; receives research support from Novartis, Boehringer Ingelheim, UCB/ SCHWARZ PHARMA, Santhera Pharmaceuticals, and Molecular Biometrics, Inc., Columbia University, Weill Cornell Medical College, Northwestern University, Indiana University, Parkinson Study Group, and the Michael J. Fox Foundation; and has served as a consultant in medico-legal cases. Dr. Marsh serves on scientific advisory boards for the National Parkinson Foundation, American Parkinson's Disease Association, and the Parkinson Study Group; receives publishing royalties for Psychiatric Issues in Parkinson's Disease: A Practical Guide (Taylor & Francis, Informa, 2005); serves as a consultant for Merck Serono, Boehringe Ingelheim, ACADIA Pharmaceuticals, and Lundbeck, Inc. (Ovation Pharmaceuticals); and receives research support from Forest Laboratories, Inc., Eli Lilly and Company, Boehringer Ingelheim, the NIH, Baylor College of Medicine, and the Michael J. Fox Foundation. Dr. Hiner has received speaker honoraria from Teva Pharmaceutical Industries Ltd. Dr. Clark reports no disclosures. Dr. Marder serves on the editorial board of Neurology; and receives research support from Amarin Corporation, Boehringer Ingelheim, NeuroSearch, the NIH, the Parkinson Disease Foundation, the Huntington's Disease Society of America, Parkinson Study Group, and the Michael J. Fox Foundation.
REFERENCES
- 1. Kitada T, Asakawa S, Hattori N, et al. Mutations in the Parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998;392:605–608 [DOI] [PubMed] [Google Scholar]
- 2. Hedrich K, Eskelson C, Wilmot B, et al. Distribution, type, and origin of Parkin mutations: review and case studies. Mov Disord 2004;19:1146–1157 [DOI] [PubMed] [Google Scholar]
- 3. Lucking CB, Durr A, Bonifati V, et al. Association between early-onset Parkinson's disease and mutations in the Parkin gene: French Parkinson's Disease Genetics Study Group. N Engl J Med 2000;342:1560–1567 [DOI] [PubMed] [Google Scholar]
- 4. Hedrich K, Marder K, Harris J, et al. Evaluation of 50 probands with early-onset Parkinson's disease for Parkin mutations. Neurology 2002;58:1239–1246 [DOI] [PubMed] [Google Scholar]
- 5. Abbas N, Lucking CB, Ricard S, et al. A wide variety of mutations in the Parkin gene are responsible for autosomal recessive parkinsonism in Europe: French Parkinson's Disease Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson's Disease. Hum Mol Genet 1999;8:567–574 [DOI] [PubMed] [Google Scholar]
- 6. Periquet M, Latouche M, Lohmann E, et al. Parkin mutations are frequent in patients with isolated early-onset parkinsonism. Brain 2003;126:1271–1278 [DOI] [PubMed] [Google Scholar]
- 7. Lohmann E, Periquet M, Bonifati V, et al. How much phenotypic variation can be attributed to Parkin genotype? Ann Neurol 2003;54:176–185 [DOI] [PubMed] [Google Scholar]
- 8. Camargos ST, Dornas LO, Momeni P, et al. Familial parkinsonism and early onset Parkinson's disease in a Brazilian movement disorders clinic: phenotypic characterization and frequency of SNCA, PRKN, PINK1, and LRRK2 mutations. Mov Disord 2009;24:662–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Macedo MG, Verbaan D, Fang Y, et al. Genotypic and phenotypic characteristics of Dutch patients with early onset Parkinson's disease. Mov Disord 2009;24:196–203 [DOI] [PubMed] [Google Scholar]
- 10. Hertz JM, Ostergaard K, Juncker I, et al. Low frequency of Parkin, tyrosine hydroxylase, and GTP cyclohydrolase I gene mutations in a Danish population of early-onset Parkinson's disease. Eur J Neurol 2006;13:385–390 [DOI] [PubMed] [Google Scholar]
- 11. Chung EJ, Ki CS, Lee WY, Kim IS, Kim JY. Clinical features and gene analysis in Korean patients with early-onset Parkinson disease. Arch Neurol 2006;63:1170–1174 [DOI] [PubMed] [Google Scholar]
- 12. Bras J, Guerreiro R, Ribeiro M, et al. Analysis of Parkinson disease patients from Portugal for mutations in SNCA, PRKN, PINK1 and LRRK2. BMC Neurol 2008;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vinish M, Prabhakar S, Khullar M, Verma I, Anand A. Genetic screening reveals high frequency of PARK2 mutations and reduced Parkin expression conferring risk for Parkinsonism in North West India. J Neurol Neurosurg Psychiatry 2010;81:166–170 [DOI] [PubMed] [Google Scholar]
- 14. Klein C, Lohmann-Hedrich K. Impact of recent genetic findings in Parkinson's disease. Curr Opin Neurol 2007;20:453–464 [DOI] [PubMed] [Google Scholar]
- 15. Klein C, Lohmann-Hedrich K, Rogaeva E, Schlossmacher MG, Lang AE. Deciphering the role of heterozygous mutations in genes associated with parkinsonism. Lancet Neurol 2007;6:652–662 [DOI] [PubMed] [Google Scholar]
- 16. Kay DM, Moran D, Moses L, et al. Heterozygous Parkin point mutations are as common in control subjects as in Parkinson's patients. Ann Neurol 2007;61:47–54 [DOI] [PubMed] [Google Scholar]
- 17. Pankratz N, Kissell DK, Pauciulo MW, et al. Parkin dosage mutations have greater pathogenicity in familial PD than simple sequence mutations. Neurology 2009;73:279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hilker R, Klein C, Ghaemi M, et al. Positron emission tomographic analysis of the nigrostriatal dopaminergic system in familial parkinsonism associated with mutations in the Parkin gene. Ann Neurol 2001;49:367–376 [PubMed] [Google Scholar]
- 19. Portman AT, Giladi N, Leenders KL, et al. The nigrostriatal dopaminergic system in familial early onset parkinsonism with Parkin mutations. Neurology 2001;56:1759–1762 [DOI] [PubMed] [Google Scholar]
- 20. Scherfler C, Khan NL, Pavese N, et al. Striatal and cortical pre- and postsynaptic dopaminergic dysfunction in sporadic Parkin-linked parkinsonism. Brain 2004;127:1332–1342 [DOI] [PubMed] [Google Scholar]
- 21. Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson's disease. Ann Neurol 2008;63:167–173 [DOI] [PubMed] [Google Scholar]
- 22. Khan NL, Katzenschlager R, Watt H, et al. Olfaction differentiates Parkin disease from early-onset parkinsonism and Parkinson disease. Neurology 2004;62:1224–1226 [DOI] [PubMed] [Google Scholar]
- 23. Verbaan D, Boesveldt S, van Rooden SM, et al. Is olfactory impairment in Parkinson disease related to phenotypic or genotypic characteristics? Neurology 2008;71:1877–1882 [DOI] [PubMed] [Google Scholar]
- 24. Marder KS, Tang MX, Mejia-Santana H, et al. Predictors of Parkin mutations in early-onset Parkinson disease: the consortium on risk for early-onset Parkinson disease study. Arch Neurol 2010;67:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Folstein MF, Folstein SE, McHugh PRP. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 26. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984;32:489–502 [DOI] [PubMed] [Google Scholar]
- 27. Doty R. The Smell Identification Test Administration Manual. Haddon Heights, NJ: Sensonics, Inc.; 1995 [Google Scholar]
- 28. Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Frequency of known mutations in early onset PD: implication for genetic counseling: the CORE-PD study. Arch Neurol (in press 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 1988;38:1237–1244 [DOI] [PubMed] [Google Scholar]
- 30. Doty RL, Riklan M, Deems DA, Reynolds C, Stellar S. The olfactory and cognitive deficits of Parkinson's disease: evidence for independence. Ann Neurol 1989;25:166–171 [DOI] [PubMed] [Google Scholar]
- 31. Hawkes C. Olfaction in neurodegenerative disorder. Mov Disord 2003;18:364–372 [DOI] [PubMed] [Google Scholar]
- 32. Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages). J Neurol 2002;249(suppl 3):III/1–5 [DOI] [PubMed] [Google Scholar]
- 33. Farrer M, Chan P, Chen R, et al. Lewy bodies and parkinsonism in families with Parkin mutations. Ann Neurol 2001;50:293–300 [DOI] [PubMed] [Google Scholar]
- 34. Pramstaller PP, Schlossmacher MG, Jacques TS, et al. Lewy body Parkinson's disease in a large pedigree with 77 Parkin mutation carriers. Ann Neurol 2005;58:411–422 [DOI] [PubMed] [Google Scholar]
- 35. Morales B, Martinez A, Gonzalo I, et al. Steele-Richardson-Olszewski syndrome in a patient with a single C212Y mutation in the Parkin protein. Mov Disord 2002;17:1374–1380 [DOI] [PubMed] [Google Scholar]
- 36. Ferraris A, Ialongo T, Passali GC, et al. Olfactory dysfunction in Parkinsonism caused by PINK1 mutations. Mov Disord 2009;24:2350–2357 [DOI] [PubMed] [Google Scholar]
- 37. Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol 2004;56:173–181 [DOI] [PubMed] [Google Scholar]
- 38. Ponsen MM, Stoffers D, Wolters E, Booij J, Berendse HW. Olfactory testing combined with dopamine transporter imaging as a method to detect prodromal Parkinson's disease. J Neurol Neurosurg Psychiatry 2010;81:396–399 [DOI] [PubMed] [Google Scholar]