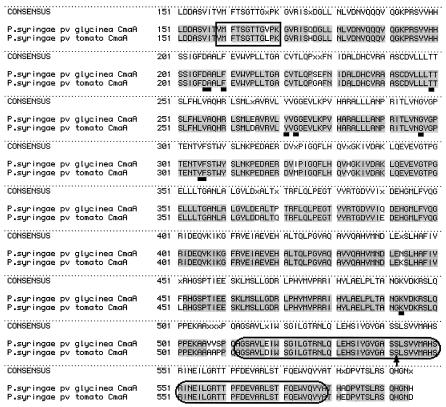
FIG. 2.
Alignment of CmaA amino acid sequences. A comparison of the CmaA sequence from P. syringae pv. glycinea with the CmaA sequence from P. syringae pv. tomato DC3000 indicates that these two sequences are 96% identical. The residues enclosed in the rectangle create an AMP-binding domain signature sequence, whereas the residues enclosed in the ellipses are common to a T domain, as determined by using the ScanProsite program at the Expasy web site. The arrow indicates the putative 4′-phosphopantetheine attachment site. The underlined residues are the residues that contribute to the specificity-conferring code of A domains in nonribosomal peptide synthetases. See the text for further discussion.