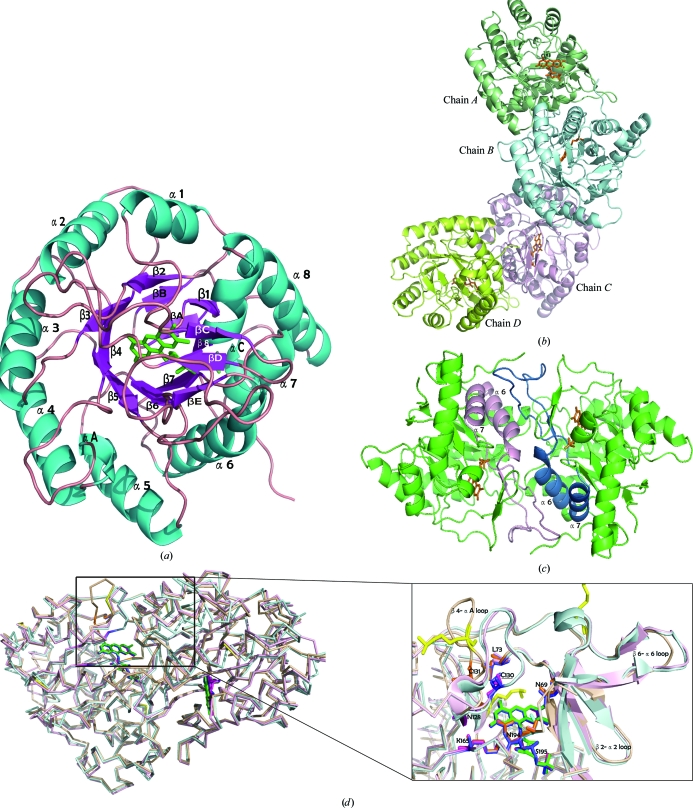
Figure 2.
Structure of SMU.595. (a) The overall crystal structure of SMU.595 is shown as a ribbon diagram with secondary-structure elements labelled in cyan (helices), violet (strands) and pink (loops). The FMN cofactor is represented as green sticks. (b) The crystal structure of the SMU.595 tetramer is shown as a ribbon diagram. The FMN cofactor is represented as orange sticks. (c) The dimer structure viewed from above the twofold axis. The β6–α6 loop and helices α6 and α7, which participate in dimer-interface interactions, are shown in pink and deep blue, respectively. The FMN cofactor is represented as orange sticks. (d) Left, structural overlay of the homodimers of SMU.595 (wheat yellow), TbDHOD (light blue) and LlDHOD (light pink). Right, the secondary structures (including the β2–α2, β4–αA and β6–α6 loops) surrounding the FMN cofactor. FMNs are shown in green, blue and pink. The amino-acid residues that are well conserved in the three sequences and that participate in interactions with FMN cofactors are shown as orange, blue and pink sticks, respectively. The methylated lysines are shown in yellow.