Figure 5.
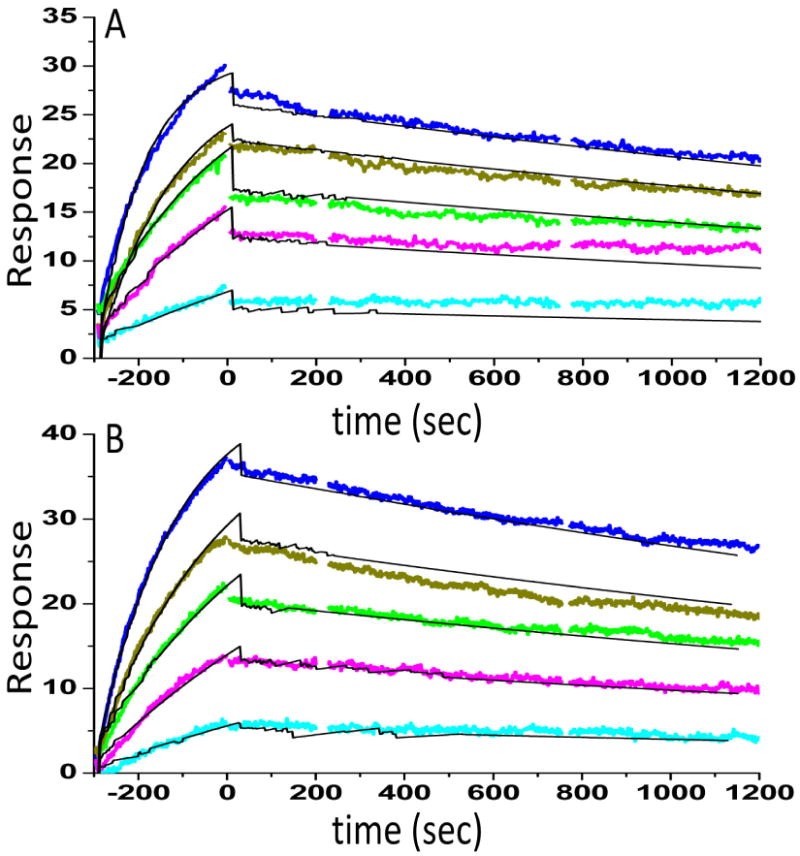
Surface plasmon resonance (Biacore) binding kinetics of the interaction between NFκB and either wild type (A) or hydroxylated (B) IκBα was performed as previously described [19]. NFκB(p50248-321/p65190–321) was biotinylated at the N-terminus of p65 and immobilized on a streptavidin sensor chip. IκBα (0.12, 0.34, 0.56, 0.95, 1.56 nM) was the flowing analyte. The data were fit using a simple 1:1 binding model yielding for the wild type protein ka = 6 × 106 M-1s-1, kd = 2.3 × 10-4, RMAX = 28.3, X2 = 0.55, and KD = 62 ±12 pM and for the hydroxylated protein ka = 4 × 106 M-1s-1, kd= 2.8 × 10-4, RMAX = 44.3, X2 = 0.74, and KD = 88 ±14 pM.
