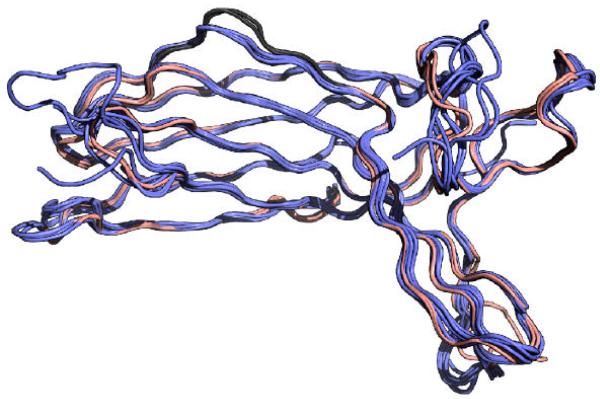
Figure 2. Arrestin-3 has a uniquely distorted C-domain element.
The N-domain element and C-domain element that are important for receptor specificity are shown in red. The residues in the arrestin-3 C-domain element that do not form proper β-sheet hydrogen bonding are shown in grey. A. The distorted region of the C-domain element is well ordered and displays clear electron density. Shown is a composite omit map drawn at 1.5 sigma. B. Overlay of arrestin-2 (1g4r, blue) and arrestin-3 (salmon), showing the deviation from standard β-sheet geometry. Distances between Cα positions in Arr2 and Arr3 are shown. C. Sausage representation of a calculated average arrestin-2 structure, based on the ten crystallographically independent arrestin-2 structures. The sausage thickness corresponds to average deviation of the eight structures from the average structure, as calculated with THESEUS 80. Receptor discriminator elements are shown in red, with the C-domain element on the left and the N-domain element on the right, showing that although the N-domain element is variable, the C-domain element in arrestin-2 is structurally conserved. D. Alignment of ten distinct arrestin-2 structures (blue, from pdbs 1g4r, 1g4m, 1zsh, 1jsy, 3gdi, 3gc3, and 2wtr) with the two arrestin-3 structures present in the asymmetric unit (salmon), indicating that all other arrestins form a contiguous β-sheet in the C-terminal domain.