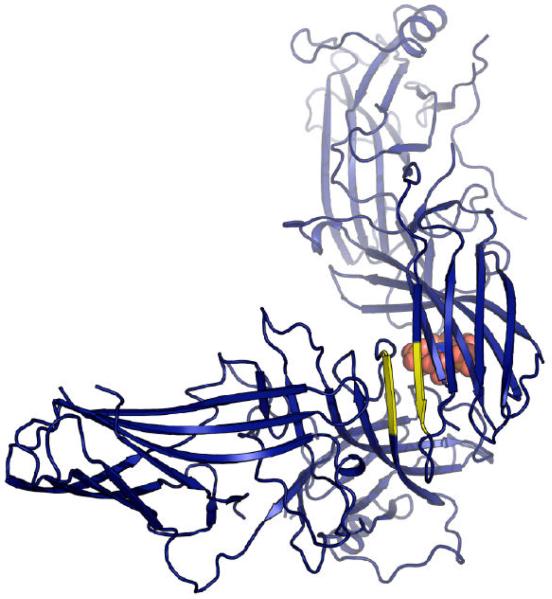
Figure 4. Conserved packing interface.
The interface (colored yellow) is a parallel ß-sheet interaction between the first strand on the concave face of the N-terminal domain and the first strand on the convex face of the C-terminal domain. The length of the interaction and the residues involved varies. A. In arrestin-1 (1cf1, green), six residues from each monomer participate, and each monomer buries ~1017Å2. B. In arrestin-2 (1g4r, light blue) three residues from each monomer participate, with each arrestin-2 monomer burying ~726Å2. C. In the arrestin-2-IP6 structure (1zsh, dark blue), four residues from each monomer participate, with each arrestin-2 monomer burying ~585Å2. IP6 is drawn as space-filling spheres and is visible in the background, behind the C-terminal β-sandwich, packed between N-terminal and C-terminal domains, in a binding site formed by dimerization. D. In arrestin-3 (3P2D, salmon), three residues from each monomer participate and each monomer buries 666 Å2.