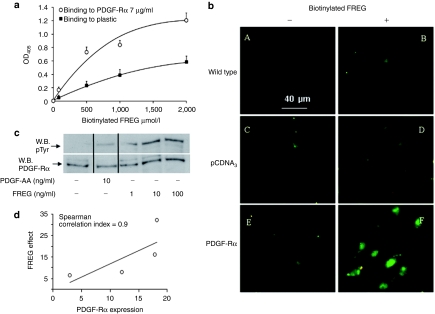
Figure 4.
FREG interacts with PDGF-Rα. (a) FREG binding to plastic-immobilized PDGF-Rα (7 µg/ml) was measured in solid-phase assays. Biotinylated FREG (form 0 to 2 mmol/l) was incubated for 4 hours onto immobilized-PDGF-Rα. Unbound material was washed away and a colorimetric assay was performed. Biotinylated FREG bound PDGF-Rα in a saturable and concentration-dependent manner. (b) SK-MEL-110 untransfected or overexpressing PDGF-Rα were seeded on glass coverslips and treated with biotinylated FREG (100 ng/ml) for 10 minutes at 4 °C and stained with Fluorescein Avidin D. Bar = 40 µm. Biotinylated FREG clearly bound to PDGF-Rα-transfected cells more than pCDNA3-transfected cells or other controls. (c) Endogenous PDGF-Rα phosphorylation was evaluated in SK-MEL-110 treated for 5 minutes with PDGF-AA (10 ng/ml) or with increasing dose of FREG (1, 10, and 100 ng/ml). PDGF-AA, used as positive control, induced phosphorylation of its selective receptor; FREG induced PDGF-Rα phosphorylation with a rate comparable to PDGF-AA. (d) Correlation between peptide effect and PDGF-Rα expression in four melanoma cell lines (namely: mouse B16F10, human SK-MEL-110, human SK-MEL-28, human MEWO). FREG was used at 100 ng/ml and its inhibitory effect on cell proliferation was correlated to PDGF-Rα expression measured by western blot analysis. Spearman correlation index was computed with PRISM 5 software for Windows. PDGF-Rα, platelet-derived growth factor-receptor-α.