Abstract
Previous studies have demonstrated that gene transfer of genes coding for neurotrophic factors to the dorsal root ganglion (DRG) using nonreplicating herpes simplex virus (HSV)–based vectors injected subcutaneously can prevent the progression of diabetic neuropathy. Because prolonged expression of neurotrophic factors could potentially have unwanted adverse effects, we constructed a nonreplicating HSV vector, vHrtEPO, to express erythropoietin (EPO) under the control of a tetracycline response element (TRE)-minimal cytomegalovirus (CMV) fusion promoter. Primary DRG neurons in culture infected with vHrtEPO express and release EPO in response to exposure to doxycycline (DOX). Animals infected with vHrtEPO by footpad inoculation demonstrated regulated expression of EPO in DRG under the control of DOX administered by gavage. Mice rendered diabetic by injection of streptozotocin (STZ), inoculated with vHrtEPO, and treated with DOX 4 days out of 7 each week for 4 weeks were protected against the development of diabetic neuropathy as assessed by electrophysiologic and behavioral measures. These studies indicate that intermittent expression of EPO in DRG achieved from a regulatable vector is sufficient to protect against the progression of neuropathy in diabetic animals, and provides proof-of-principle preclinical evidence for the development of such vectors for clinical trial.
Introduction
Polyneuropathy is a common and often debilitating complication of diabetes for which no effective treatments are currently available. In animal models of diabetic neuropathy, systemic delivery of any one of a number of different trophic factors has been shown to prevent progression or reverse the signs of neuropathy,1,2,3 but translation of systemic trophic factor therapy to the treatment of human diseases has not succeeded. Gene transfer offers a potential solution to the problems inherent in attempting to deliver an adequate dose of short-lived peptide factors with pleiotropic off-target effects to sensory neurons of the peripheral nervous system. Toward this end, we have developed a series of recombinant, replication-incompetent, genomic herpes simplex virus (HSV)–based vectors for gene transfer to the nervous system. In the mouse model of type 1 diabetes created by injection of streptozotocin (STZ), transduction of dorsal root ganglion (DRG) by subcutaneous inoculation of HSV vectors expressing nerve growth factor, neurotrophin-3, vascular endothelial growth factor, or erythropoietin (EPO) each provide protection against the development of sensory and autonomic peripheral nerve dysfunction resulting from diabetes.4,5,6,7 Prolonged expression can be achieved by the use of an HSV-based vector in which transgene expression is controlled by the HSV latency promoter (LAP2) element. An HSV vector expressing neurotrophin-3 under the regulatory control of the LAP2 promoter element prevents the development of sensory and autonomic dysfunction up to 6 months in STZ-diabetic mice.5
But because polyneuropathy is a chronic condition and there may be potential adverse affects from long-term expression of potent neurotrophic factors within the peripheral sensory nerves, a critical step required before HSV gene therapy for neuropathy that can be moved from preclinical animal models into human trials is the development of vectors from which gene expression may be regulated by exogenously administered drugs. A number of different regulatable systems have been established, including Tet-on, Tet-off, and rapamycin-based constructs.8,9,10,11,12,13,14 Effective regulation of transgene expression has been demonstrated in the retina, salivary gland, liver, kidneys, and brain.15,16,17,18,19,20,21 In this study, we constructed a nonreplicating HSV vector to express EPO under the control of a Tet-on system. Effective regulation of EPO expression was demonstrated in primary DRG neurons in culture and in DRG in vivo following subcutaneous inoculation of an EPO-expressing HSV vector into the foot. Intermittent doxycycline (DOX)-regulated expression of EPO was sufficient to prevent the progression of diabetic neuropathy in mice with STZ-induced diabetes. Taken together, these results provide important preclinical data for the development of a regulatable system for the treatment of diabetic polyneuropathy.
Results
Regulatable expression vector construct
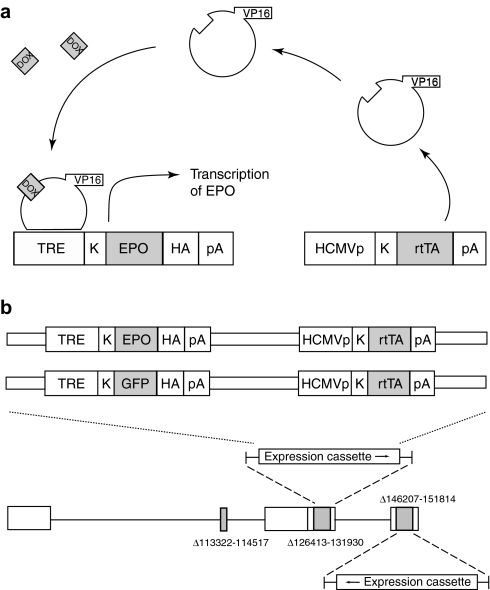
Regulation of expression was achieved using the Tet-on system tetracycline response element (TRE)-Tight. This commercially available system consists of two plasmids: the first coding for a hybrid transactivator consisting of an Escherichia coli TetR protein DOX and DNA-binding domains fused to the activation motif of the HSV VP16 protein and the second consisting of a Tet response element—minimal cytomegalovirus (CMV) fusion promoter upstream of the transgene insertion site. In the presence of DOX, conformational changes in the DNA-binding domain in the regulatable transactivator allow the transactivator to bind to the TRE of the inducible fusion promoter in the transgene expression cassette, whereupon VP16 drives expression of the transgene (Figure 1a). A pair of expression cassettes containing both the regulatable transactivator under the control of an HCMV IEp and the gene for either EPO or green fluorescent protein [GFP; each fused to an hemagglutinin (HA) sequence] under the control of a minimal CMV promoter downstream of the TRE were constructed, and inserted by homologous recombination to replace both copies of ICP4 in the nonreplicating HSV-1 recombinant UL41E1G6 (Figure 1b). Two final vectors were isolated and purified: vHrtEPO containing the gene for rat EPO fused to an HA sequence and the control vector vHrtGFP containing the gene for GFP fused to an HA sequence.
Figure 1.
Regulatable HSV vector. (a) Schematic representation of the two-element based Tet-on system employed in these studies. (b) Schematic representation of the HSV vector constructs. Two copies of the expression cassette containing both components of the TRE-Tight system were inserted in to the ICP4 locus of the parental recombinant UL41E1G6. DOX, doxycycline; EPO, erythropoietin; HA, hemagglutinin; rtTA, reverse Tet-controlled transactivator; TRE, tetracycline response element.
vHrtEPO expresses EPO under the control of DOX in vitro
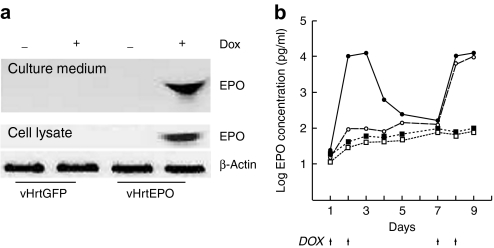
We first tested DOX-responsive regulation of EPO expression in primary DRG neurons in vitro. DRG neurons 7 days after seeding were infected with either vHrtEPO or control vector vHrtGFP at a multiplicity of infection of 1 and 24 hours later exposed to DOX (10 mg/ml, which is the minimal concentration required for the maximal expression of EPO determined in preliminary experiments; data not shown) for 48 hours. Neurons infected with vHrtEPO and exposed to DOX expressed EPO detectable in cell lysates and culture medium by western blot (Figure 2a). There was no detectable expression of the EPO in cells infected with control vector vHrtGFP, or in cells transduced with vHrtEPO but not exposed to DOX (Figure 2a). We examined the kinetics of expression by measuring the amount of EPO released into the medium from infected cells using DOX on–off–on and off–on paradigms. A robust increase in EPO in the medium was observed when vHrtEPO-infected cells were exposed to DOX for 2 days (Figure 2b, closed circles; note that the y-axis is plotted on a logarithmic scale). Removal of DOX resulted in a rapid decrease in EPO concentration in the medium. Re-exposure of the cells to DOX at days 7 and 8 restored EPO to a level that was essentially indistinguishable from that produced by first exposure to DOX (Figure 2b). Cells infected with vHrtEPO and exposed to DOX on days 7 and 8 after infection showed a substantial and statistically significant increase in EPO released into the medium measured on days 8 and 9 (Figure 2b, open circles). No detectable expression of EPO was seen in cells infected with control vector vHrtGFP and treated with DOX in a similar fashion on either days 1 and 2 or days 7 and 8.
Figure 2.
Induction of EPO expression in vitro. (a) Western blot of the culture medium (top) and cell lysate (bottom) from DRG neurons infected with vHrtGFP or vHrtEPO at an MOI of 1 for 48 hours, with or without DOX treatment (+, − above blot). (b) Kinetics of EPO release into the medium of primary DRG neurons transduced by vHrtEPO (circles) or vHrtGFP (squares). Cells were treated with DOX (arrows) on days 7 and 8 (open symbols) or on days 1, 2, 7, and 8 (filled symbols). DOX, doxycycline; EPO, erythropoietin.
vHrtEPO expresses EPO under the control of DOX in vivo
In order to examine regulation of EPO expression from the vector by DOX in vivo, mice were injected with vHrtEPO (4 × 108 plaque-forming units in 10 µl phosphate-buffered saline) into the plantar surface of both hind feet. Three days after virus injection, DOX (0.2 ml of 10 mg/ml)22 was administered by gavage daily, and cohorts of animals killed at 1, 2, 3, and 4 days. Increasing amounts of EPO mRNA (Figure 3a) and protein (Figure 3b) were found in DRG of the vector-inoculated and DOX-treated animals. The amount of EPO mRNA and protein increased over 4 days of treatment. Animals inoculated with vHrtEPO but not treated with DOX showed no detectable EPO mRNA or protein in the DRG (Figure 3c,d). To analyze the kinetics of shutoff in the absence of DOX in vivo, animals were inoculated with vHrtEPO and treated with DOX by gavage for 4 days, after which DOX treatment was discontinued and the animals allowed to survive for an additional 3 days. Animals receiving 4 days of DOX followed by 3 days of no treatment showed no detectable EPO mRNA or protein in the DRG (Figure 3e,f) similar to that observed in animals inoculated with the vector but not treated with DOX. Quantitative analysis of the amount of EPO mRNA and protein (normalized to β-actin) showed a substantial increase in EPO expression at both mRNA and protein levels in DRG in vivo (Figure 4a,b) with the protein lagging slightly behind the increase in mRNA as might be anticipated. Quantitative assessment of the amount of EPO in the DRG using an enzyme-linked immunosorbent assay (ELISA) showed an increase from <5 pg/µg protein (about the detection limit of the ELISA kit) in naive animals to >140 pg/µg protein in animals inoculated with vHrtEPO after 4 days of treatment with DOX (Figure 4c).
Figure 3.
Regulation of EPO expression in vivo by DOX. Expression of (a) EPO mRNA (semiquantitative reverse transcription-PCR) and (b) protein (detected by western blot using an antibody against the hemagglutinin tag in dorsal root ganglion of mice treated with doxycycline (DOX) by gavage for 4 days. Animals inoculated with the vector but not treated with DOX show no detectable expression of EPO by semiquantitative reverse transcription(RT)-PCR (−, c) or western blot (−, d), compared to animals inoculated with the vector and gavaged with DOX for 4 days (+, c,d). Animals treated with DOX for 4 days, followed by 3 days without DOX show no detectable expression of EPO mRNA by semiquantitative RT-PCR (+/−, e) or western blot (+/−, f), similar to animals inoculated with the vector and never treated with DOX (−, e,f). EPO, erythropoietin.
Figure 4.
Time course of EPO expression in vivo. Quantitative analysis of (a) EPO mRNA and (b) protein levels in DRG, and (c) quantification of EPO by ELISA in dorsal root ganglion of animals treated with daily DOX gavage as indicated by arrows. DOX, doxycycline; ELISA, enzyme-linked immunosorbent assay; EPO, erythropoietin.
DOX-induced expression of EPO from the vector prevents the progression of diabetic neuropathy in vivo
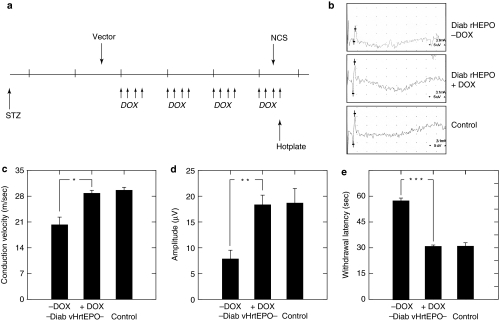
To determine whether regulated expression of EPO would preserve peripheral nerve function in diabetic animals, we tested the vector in a mouse model of diabetic polyneuropathy. Male Swiss Webster mice were rendered diabetic by intraperitoneal injection of STZ, and 2 weeks later inoculated with vHrtEPO (4 × 108 plaque-forming units in 10 µl phosphate-buffered saline) into the plantar surface of both hind feet. Three days after vector inoculation animals were treated with either DOX (0.2 ml of 10 mg/ml) or water by gavage. Preliminary to the therapeutic trial, we determined that regulated expression of EPO in the DRG of diabetic animals was identical to that which we had observed in control animals (Figure 5). In the therapeutic test, DOX treatment by gavage was carried out for 4 consecutive days followed by 3 days off each week (Figure 6a), and after 4 weeks of treatment, sensory nerve amplitude and velocity were determined by nerve conduction studies (Figure 6b), followed the next day by determination of thermal threshold using the hot plate test. Diabetic animals inoculated with vHrtEPO and treated with water by gavage showed significantly reduced sensory nerve amplitudes and conduction velocity (Figure 6c,d) consistent with neuropathy,4,7 but diabetic animals inoculated with vHrtEPO and treated with DOX by gavage had amplitudes and conduction velocities indistinguishable from control, nondiabetic mice. Diabetic animals inoculated with vHrtEPO and treated with water by gavage had a significantly increased latency to withdraw from a painful thermal stimulus (Figure 6e), but diabetic mice inoculated with vHrtEPO and treated with DOX had a normal withdrawal latency that was identical to that of control, nondiabetic mice.
Figure 5.
Regulated EPO expression in diabetic mice. Diabetic mice inoculated with the vector but not treated with doxycycline (DOX) show no detectable expression of EPO by semiquantitative reverse transcription (RT)-PCR (−, a) or western blot (−, b), compared to animals inoculated with the vector and gavaged with DOX for 4 days (+, a,b). Diabetic mice treated with DOX for 4 days, followed by 3 days without DOX show no detectable expression of EPO mRNA by semiquantitative RT-PCR (+/−, c) or western blot (+/−, d), similar to animals inoculated with the vector and never treated with DOX (−, c,d). EPO, erythropoietin.
Figure 6.
Effect of regulated EPO expression on diabetic neuropathy. (a) Schematic of treatment protocol. Diabetes was induced by injection of streptozotocin (STZ). Two weeks later vHrtEPO was inoculated subcutaneously in both hind feet. Doxycycline (DOX) was administered by gavage 4 days out of 7 (arrows) every week for 4 weeks. Nerve conduction studies (NCS) and hotplate testing was performed at the end of the fourth week of treatment. (a) DOX treatment on/off schedule. (b) Representative recordings. (c) Nerve conduction velocity, (d) amplitude, and (e) withdrawal latency from painful thermal stimulation in naive and vHrtEPO-infected animals treated with or without DOX. *P < 0.005; **P < 0.001; ***P < 0.0001.
Discussion
The principal results of this study demonstrate that (i) expression of EPO by DRG neurons in vitro and in vivo infected with vHrtEPO is under the relatively tight control of administration of DOX, and (ii) intermittent expression of EPO, 4 days out of 7 each week for 4 weeks is sufficient to prevent the progression of sensory neuropathy in diabetic mice.
Several studies have defined some of the molecular mechanisms through which EPO can protect nerve from degeneration in models of diabetes-related and other neuropathies.4,23,24,25 However, the ability to regulate gene expression will be critical for human gene therapy applications in which prolonged transgene expression is required. Local expression of a neurotrophic factor in sensory neurons of the DRG to protect the peripheral nerve degeneration will reduce the potential for off-target effects, as demonstrated by the observation that HSV-mediated expression of EPO in DRG at levels sufficient to prevent diabetic neuropathy does not increase hematocrit.4 But the possibility of unanticipated adverse effects is not eliminated by local delivery. For example, continuous expression of EPO might lead to the development of abnormalities in the vascular supply to the in nerve. One approach to this potential problem would be to use a system that provides transient transgene expression coupled with reinoculation of the vector at intervals. Although reinoculation of HSV vectors can be used to reestablish transgene expression in rodent models of peripheral neuropathy and pain, such a system would be cumbersome and the upper limit on reinoculation is not established. Several systems are available in which transgene expression from a vector may be shut off by drug administration.8,9,14 However, separate from the problem of leaky expression that may occur in the presence of the drug, the practical application of such a system for human treatment would be problematic. Compliance would likely be a problem in patients who experienced a problem with the gene therapy and would then be required to take the drug continuously to prevent the off-target effects. And although HSV is a nonintegrating vector, it establishes a lifelong persistent state as an intranuclear episomal element in the sensory neurons of the DRG making removal of the vector not feasible.
Several different regulatable systems to turn on gene expression have been developed.13,14 We chose to use a Tet-on system because DOX is a drug that is already approved for human use. The modified TRE-Tight system has tightly regulated expression in the presence of DOX with virtually no background.9,10 The in vitro studies in primary DRG neurons demonstrate a barely detectable background activity in the absence of DOX. We combined the two plasmids that make up the TRE-Tight system into a single HSV vector in order to maximize the opportunity for regulated gene expression in vivo, where the percentage of the DRG neurons that are infected may be small. The data in vivo demonstrate robust regulation of transgene expression by DOX. Three lines of evidence indicate that regulation of transgene expression from the vector by DOX is relatively tight. There was very little EPO mRNA detectable by 20 cycles of semiquantitative reverse transcription-PCR, and essentially no detectable EPO protein by western blot under conditions in which DOX-stimulated production was easily detected. Moreover, the nerve conduction velocity and amplitude and the latency to withdraw from a thermal stimulus in diabetic animals inoculated with the vector but not treated with DOX showed no evidence of EPO-mediated protection of nerve function.
In this study, we administered DOX by gavage at a dose of 66 mg/kg body weight, based on previous reports.22 We chose to administer DOX by gavage because the requirement to supplement drinking water with 1% sucrose to mask the taste of the drug would exacerbate the hyperglycemia and polyuria in these animals and is therefore not appropriate. However, in other studies, it has been demonstrated that DOX at a dose of 5 or 6 mg/kg body weight in drinking water is sufficient to activate gene expression using a Tet-on system in vivo in rats and in monkeys26,27 indicating that DOX at doses appropriate for clinical trials is likely to be effective for this purpose.
There are many different models of diabetic neuropathy in mouse, rat, and rabbit. None of these models perfectly replicate the human disease,28,29 but we chose to use the STZ-diabetic mouse because (i) these animals show loss of large and small fibers, indicated by slowing of nerve conduction velocity and loss of skin innervations respectively, and (ii) despite persistent hyperglycemia the animals are not overtly ill and therefore do not require supportive treatment. We chose in this first proof-of-principle study to drive expression of the transactivator using the HCMV IEp, because we have previously demonstrated that HCMV-driven expression of EPO from an HSV vector is effective in preventing the emergence of neuropathy in diabetic mice using the experimental paradigm employed in this study.4 In this study, we found that intermittent EPO expression mediated by 4 days of DOX treatment every 7 days for 4 weeks was sufficient to prevent the emergence of neuropathy in these animals. It is certainly possible that a shorter administration of DOX, or more days off between DOX doses might be able to achieve the same results. Such practical considerations will be important to evaluate before designing a clinical trial of regulated transgene expression and for prolonged transgene expression, it will be preferable to drive transactivator expression using the LAP2 promoter element that has been demonstrated to be capable of providing at least 6 months of biologically meaningful transgene expression in the DRG.5 The next step toward a clinical trial will be studies using different regimens of DOX administration (e.g., less administrations per week or less weeks per month) over a prolonged course of diabetes using the LAP2 vector.
Because HSV-mediated gene transfer to the DRG is already in phase 1 clinical trial using a related nonreplicating vector expressing preproenkephalin in patients with chronic intractable pain related to cancer, successful completion of that phase 1 trial coupled with the development of a LAP2 driven regulatable vector may set the stage for a clinical trial of HSV-mediated gene therapy for the treatment of diabetic neuropathy.
Materials and Methods
Cells and viruses. African green monkey kidney cells (Vero) were maintained and grown in Dulbecco's modified Eagle's essential medium supplemented with 10% fetal bovine serum (Atlanta Biologics, Atlanta, GA), 100 units/ml penicillin, 100 µg/ml streptomycin sulfate, 0.03% glutamine, and 0.375% sodium bicarbonate in a 5% CO2 atmosphere. The 7b cells (Joseph Glorioso, University of Pittsburgh), a derivative of Vero cells that expresses HSV-1 ICP27 and ICP4, were maintained and propagated in 10% fetal bovine serum–containing Dulbecco's modified Eagle's essential medium.
DRG neurons were dissociated from DRGs dissected from 17-day rat embryos by 0.25% trypsin plus 1 mmol/l EDTA at 37 °C for 30 minutes. A volume of 1.5 × 105 cells were plated in poly--lysine-coated 24-well plates and cultured in Neurobasal medium (Invitrogen, Carlsbad, CA) supplemented with B27, Glutamax I, Albumax, Pstrep, and 7.0S nerve growth factor. Schwann cells were reduced from the DRG culture by addition of 5′-fluoro-2′-deoxy-uridine/uridine mixture twice with a working concentration of 0.125 mg/ml for each and DRG cells cultured with 60% medium change every other day.
The parental HSV vector UL41E1G6 (provided by Joseph Glorioso, University of Pittsburgh) used for generation of the recombinant viruses in this study is an HSV-1 null mutant deleted for the essential gene ICP27 with both copies of the IE gene ICP4 replaced by hCMV-GFP. UL41E1G6 was propagated in complementing 7b cells and the virus titer was determined by plaque assay.30
Construction of plasmids pSASB3-EPO-HA and pSASB3-GFP-HA. Plasmid pSASB3-EPO-HA was constructed as follows. The full-length rat EPO was amplified from rat kidney complementary DNAs by PCR with the forward primer containing an EcoRI recognition site and Kozak sequence at the 5′-end and the reverse primer possessing an XbaI site. The sequences of the primers are as follows, the forward primer: 5′-CCGGAATTCGCCAGGCGCGGAGATG-3′, and the reverse primer: 5′-TGCTCTAGACGTGACACACAGTGACGGTA-3′. The PCR product was cloned into a PCR cloning plasmid pGEM-T (Promega, Madison, WI) to generate plasmid pGEM-T-EPO. The BsrGI–XbaI fragment in pGEM-T-EPO was replaced by an annealed oligoduplex, which contains a BsrGI sticky overhang at the 5′-end, a small portion of EPO 3′-terminal coding sequence, HA tag, a stop codon, and an XbaI sticky end sequence at 3′-end. The sequences of the upper and lower oligos in the duplex are as follows, the upper: 5′-GTACACGGGGGAGGCCTGCAGGAGAGGGGA CAGGTACCCATACGATGTTCCAGATTACGCTTGAT-3′, The lower: 5′-CTAGATCAAGCGTAATCTGGAACATCGTATGGCTA CCTGTCCCCTCTCCTGCAGGCCTCCCCCGT-3′. The resulting plasmid pGEM-EPO-HA contains the full-length EPO in-frame fused with an HA tag at the C-terminus of EPO. EPO-HA fragment was amplified by PCR with an upper prime containing an EcoRI recognition site and a lower primer possessing a BamHI site to replace the XbaI site located immediately downstream of the EPO-HA by BamHI. The upper and lower primers are 5′-CCGGAATTCGCCAGGCGCGGAGATG-3′ and 5′-CGGGATCCTCAAGCGTAATCTGGAACATC-3′, respectively. The PCR product was cloned into the EcoRI and BamHI sites of plasmid pTRE-Tight (Clontech, Mountain View, CA) to get plasmid pTRE-Tight-EPO-HA, in which EPO expression is under the control of the inducible promoter containing a Tet-responsive element and a minimal CMV promoter. To generate a functional regulatable EPO expression unit with the transactivator-expression element and the regulatable EPO expression cassette in one plasmid, an engineered pSP72 plasmid pSP72-linker was created by replacing the multiple cloning sites in pSP72 (Promega) by a polylinker containing restriction sites XhoI (blocked)-BglII-XhoI-HindIII-SalI-EcoRI as a transition plasmid. The sequences of the linker upper and lower oligos are as follows, the upper: 5′-TCGACAGATCTCTCGAGCGGCCCAAGCTTGTCGACG-3′, and the lower: 5′-AATTCGTCGACAAGCTTGGGCCGCTCGAGAGATCTG-3′.The full-length transactivator-containing fragment was excised from plasmid Tet-on (Clontech) and cloned into the XhoI and HindIII sites of pSP72-linker resulting in plasmid pSP72-link-Tet-on. The inducible EPO expression cassette, which possesses all elements required for EPO expression driven by transactivator in the presence of DOX, was cut from pTRE-Tight-EPO-HA by XhoI cleavage and ligated into the XhoI site of pSP72-linker-Tet-on. The resulting plasmid pSP-72-link-TRE-Tight-EPO-HA-Tet-on contains a complete EPO-inducible expression unit. The whole EPO-inducible expression unit was released from pSP-72-link-TRE-Tight-EPO-HA-Tet-on by BglII cleavage and cloned into the BamHI site of plasmid pSASB3 (Joseph Glorioso, University of Pittsburgh) for facilitating the construction of HSV-1 based EPO expression vector.
Plasmid pSASB3-GFP-HA, which inducibly expresses green fluorescence protein-HA fusion in the presence of DOX, was generated in similar ways used for generation of pSASB3-EPO-HA except for the initial steps. GFP was amplified by PCR from plasmid pGFP (Clontech) with the forward primer containing an EcoRI recognition site and Kozak sequence at the 5′-end and the reverse primer possessing a NotI site at the 5′-end. The primers are as follows, the upper: 5′-CGGAATTCCGCCACCATGGCTAGCAAAGGAG-3′, and the lower: 5′-ATAGTTTAGCGGCCGCTTTGTAGAGCTCATCC-3′. The resulting PCR fragment was ligated into the EcoRI and NotI sites of pTRE-Tight to get plasmid pTRE-Tight-GFP. The NotI to BamHI fragment in pTRE-Tight-GFP was then replaced by an HA tag—containing oligoduplex. The sequences of the upper and lower oligos in the duplex are as the follows, the upper: 5′-GGCCGCATACCCATACGATGTTCCAGATTACGCTTGAT-3′ and the lower: 5′-CTAGATCAAGCGTAATCTGGAACATCGTATGGGTATGC-3′. The resultant plasmid expresses GFP–HA fusion protein with three alanines stuffed in between GFP ORF and HA tag, and the extra nucleotide sequence encoding the three alanines was added to ensure that the HA tag was in-frame fused to GFP. The remaining steps for completing pSASB3-GFP-HA construction were conducted as exactly the same as described for generation of pSASB3-EPO-HA.
Construction of regulatable EPO and GFP expression vectors. Recombinant HSV-1 vectors vHrtEPO expressing rat EPO-HA and hHGFP expressing GFP–HA fusion proteins in the presence of DOX, respectively, were generated based on homologous recombination between plasmid pSASB3-EPO-HA or pSASB3-GFP-HA and replication-deficient HSV-1 genome DNA (UL41E1G6) in complementing 7b cells. The regulatable gene expression unit in plasmids pSASB3-EPO-HA and pSASB3-GFP-HA was flanked by sequences corresponding to the up- and downstream sequences of the ICP4 open reading frame in HSV-1 at the 5′- and 3′-ends, respectively, which serve as the basis for homologous recombination between the plasmid DNA and the viral genome.31 To generate the HSV-1-based recombinant vector, 3.5 × 105 7b cells in Pstrep-free Dulbecco's modified Eagle's essential medium were seeded into 6-well plates and incubated at 37 °C in an atmosphere of 5% CO2 overnight. Cells were infected with UL42E1G6 at multiplicities of 3, 1, 0.5, and 0.1 plaque-forming units per cell, followed 1 hour later by transfection with 2 µg of pSASA3-EPO-HA or pSASB3-GFP-HA. Transfection medium was replaced by complete Dulbecco's modified Eagle's essential medium 4 hours after transfection and cells incubated in a CO2 incubator allowing for virus to grow. Cells were harvested when at least 90% of cells showed cytopathic effects and viruses released from cells by three cycles of freeze and thaw. Subsequent recombinant virus screening and purification were conducted using the methods as described previously.31 The identities of the recombinant viruses were confirmed by PCR amplification of the transgenes followed by DNA sequencing. The EPO and the control GFP vectors were also confirmed by induced expression of EPO or GFP in Vero cells by DOX. Recombinant viruses were propagated in 7b cells and purified by Opti-prep gradient centrifugation. Purified viruses were aliquoted and stored at −80 °C until use.
Animal experiments. Male Swiss Webster mice weighing 20–25 g (Charles River, Wilmington, MA) were used for all the experiments. All animal procedures in this study were performed in compliance with approved institutional animal care and use protocols. For analyzing regulation of EPO expression from the vector by DOX, mice were inoculated with 2 × 108 plaque-forming units vHrtEPO in 10 µl phosphate-buffered saline buffer in the plantar surface of both hind feet. Three days after vector injection, animals were treated with DOX (0.2 ml of 10 mg/ml) by gavage on a daily basis and DOX administration lasted for 4 consecutive days. Five animals were killed each day during DOX treatment and L4-6 DRGs removed for EPO mRNA and protein assays. To examine whether stoppage of DOX treatment turns off the EPO expression from the vector induced by DOX in animals, animals were infected by the vector vHrtEPO and treated by DOX for 4 consecutive days after vector injection, and after which left alone without DOX treatment for 3 days, then killed and L4-6 DRGs removed for EPO expression assays. Animals gavaged with 0.2 ml water served as the controls during the experiments. To analyze whether induction of EPO expression from the vector by DOX is altered in diabetic animals, mice were rendered diabetic by STZ injection twice in 2 days with a dose of 100 mg per kg animal weight.32 Blood glucose in the animals was measured 2 weeks after STZ injections and the animals with blood glucose concentration higher than 300 mg/dl selected for use. Diabetic (test group) and normal (positive control group) animals were injected with vHrtEPO and DOX administrated to animals 3 days after vector injection. A portion of animals in each group were killed after 4-day DOX or vehicle (water) treatment and L4-6 DRGs removed. The remaining animals were left alone without any treatment for additional 3 days and then killed and DRGs removed for EPO expression assays. To determine whether regulatable expression of EPO from the vector preserves sensory nerve functions in diabetic animals, diabetic animals were infected by the EPO vector 2 weeks after STZ injections and treated with DOX (10 animals) or the vehicle water (10 animals) for 4 weeks with a weekly administration schedule of 4-day on/3-day off. Sensory nerve conduction velocity and the sensitivity of animals to a heat stimulus were measured at the day of the last DOX administration at the fourth week of DOX treatment or 1 day before, respectively. Animals were killed 1 day after the last DOX treatment at the 4th week of DOX treatment and L4-6 DRGs removed for EPO mRNA and protein assays.
Electrophysiological testing. Sensory nerve recordings were performed on the right hind foot using a Nicolet Viking III EMG device (Nicolet Biomedical, Madison, WI). Mice were anesthetized with isoflurane for testing, and subcutaneous temperature maintained at 36–37 °C. The hindlimbs were secured at an angle of 30° relative to the body and a ground electrode inserted into the tail, electrodes inserted into the sciatic notch as the recording electrode, while the stimulating electrode was placed at the ankle and the reference electrode was positioned at the first digit.
Hotplate test. The sensitivity of animals to heat stimulus was evaluated by hotplate test on a metal plate (UgoBasile, Comeria, Italy). The initiation temperature was set at 47 °C and programmed to increase at 2 °C/minutes. Withdrawal latency was measured in seconds to the time the animals lifted their hind paw from the plate or licked the paw. The cutoff temperature was 49 °C. An animal was placed onto the plate when it was heated to 47 °C, and testing for each animal was conducted in triplicate with a time interval of at least 5 minutes between tests.
Western blot. Mouse L4-6 DRGs were homogenized in NP-40 lysis buffer containing 50 mmol/l Tris, 10 mmol/l NaCl, 1% Nonidet P-40, 0.02% NaN3, and 1: 100 dilution of protease inhibitor and phosphatase inhibitor cocktails (Sigma, St Louis, MO). Cultured rat DRG cells were collected in the same buffer after being dislodged from the culture plates with a cell scraper. Tissue homogenates and cell lysates were sonicated and the mixture clarified by centrifugation at 10,000 r.p.m. for 10 minutes at 4 °C. Protein concentration was measured using the BCA assay (Pierce, Rockford, IL). For western blotting detection of EPO in medium from DRG culture, medium was concentrated tenfold by filtration using Centricon columns (Millipore, Bedford, MA). A 20 µg protein or 30 µl concentrated medium per lane were mixed with Laemmli buffer and denatured at 95 °C for 5 minutes before being separated by 10% Tris–glycine sodium dodecyl sulfate-polyacrylamide gel electrophoresis, after which they were transferred to an Immobilon-P membrane (0.45 µm; Millipore). The blots were blocked with 5% nonfat milk, and then incubated with mouse anti-HA antibody (1:1,000; Sigma) followed by incubation with horseradish peroxidase-conjugated anti-mouse IgG (1:2,000; Santa Cruz Biotechnologies, Santa Cruz, CA). Protein bands were visualized with enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).The membranes were stripped and reprobed with anti-β-actin antibody (1:2,000; Sigma) as a loading control. The intensity of each band was determined using a PC-based image analysis system (ChemiDoc XRS System; Bio-Rad, Hercules, CA).
EPO ELISA. The amount of EPO was determined by ELISA using an EPO ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The primary antibody in the kit reacts with both rat and mouse EPO. 50 µl of medium or 1–5 µg protein was utilized for EPO assays in medium or cell lysates, respectively. All ELISA assays were conducted in triplicates and the results expressed as mans ± SD.
RNA isolation and reverse transcription. Tissues were homogenized in lysis buffer and total RNA from tissue homogenates or cell lysates isolated using the RNeasy plus kit from Qiagen (Valencia, CA) according to the manufacturer's instructions. RNA quality was monitored by agarose electrophoresis and concentration measured using a UV spectrophotometer. Reverse transcription was conducted using the Superscript reverse transcriptase II kit from Invitrogen. The RNA input was 2 µg and poly-T used as the primer for reverse transcription. The synthesized complementary DNA was used for gene amplification by PCR or used for quantification of mRNA levels by semiquantitative PCR.
PCR. PCR amplification was carried out in 50 µl using a standard protocol with an initial denaturing step at 94 °C for 5 minutes followed by either 35 (DNA amplification for cloning or identification of gene identity) or 20 cycles (semiquantitative PCR for quantifying mRNA levels) at 95 °C, 1 minutes/60 °C, 30 seconds/72 °C, 1 minutes and the products separated on a 0.8% agarose gel. The primers used in PCRs are as follows. For rat EPO amplification from rat kidney complementary DNAs for EPO cloning, the forward primer is 5′-CCGGAATTCGCCAGGCGCGGAGATG-3′ and the reverse primer is 5′-TGCTCTAGACGTGACACACAGTGACGGTA-3′. For identification of vHrtEPO identity or semiquantification of exogenous EPO expression, the forward primer is 5′-CCGGAATTCGCCAGGCGCGGAGATG-3′ and the reverse primer is 5′-CGGGATCCTCAAGCGTAATCTGGAACATC-3′. For GFP amplification for cloning, the forward primer is 5′-CGGAATTCCGCCACCATGGCTAGCAAAGGAG-3′ and the reverse prime is 5′-ATAGTTTAGCGGCCGCTTTGTAGAGCTCATCC-3′. For identification of vHrtGFPhHGFP identity, the forward prime is 5′-CGGAATTCCGCCACCATGGCTAGCAAAGGAG-3′ and the reverse primer is 5′-CTAGATCAAGCGTAATCTGGAACATCGTATGGGTATGC-3′. For semiquantification of actin mRNA levels, the forward primer: 5′-CAGTTCGCCATGGATGACGATATC-3′, and the reverse primer: 5′-CACGCTCGGTCAGGATCTTCATG-3′.
Statistical analysis. The statistical significance of the difference between groups was determined by Student t-test and results expressed as means ± SD.
Acknowledgments
This work was supported by grants from the Juvenile Diabetes Research Foundation, the National Institutes of Health and the Department of Veterans Affairs to D.J.F. and M.M. We acknowledge the excellent technical assistance of Vikram Thakur in propagation of the vectors, the help of Shue Liu in DRG cell culture and the advice of Munmun Chattopadhyay for electrophysiologic testing and behavioral studies.
REFERENCES
- Pradat PF, Kennel P, Naimi-Sadaoui S, Finiels F, Orsini C, Revah F, et al. Continuous delivery of neurotrophin 3 by gene therapy has a neuroprotective effect in experimental models of diabetic and acrylamide neuropathies. Hum Gene Ther. 2001;12:2237–2249. doi: 10.1089/10430340152710577. [DOI] [PubMed] [Google Scholar]
- Wellmer A, Misra VP, Sharief MK, Kopelman PG., and, Anand P. A double-blind placebo-controlled clinical trial of recombinant human brain-derived neurotrophic factor (rhBDNF) in diabetic polyneuropathy. J Peripher Nerv Syst. 2001;6:204–210. doi: 10.1046/j.1529-8027.2001.01019.x. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Riekhof JT., and, Wright DE. Restorative effects of neurotrophin treatment on diabetes-induced cutaneous axon loss in mice. Exp Neurol. 2003;179:188–199. doi: 10.1016/s0014-4886(02)00017-1. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Walter C, Mata M., and, Fink DJ. Neuroprotective effect of herpes simplex virus-mediated gene transfer of erythropoietin in hyperglycemic dorsal root ganglion neurons. Brain. 2009;132:879–888. doi: 10.1093/brain/awp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M, Wolfe D, Mata M, Huang S, Glorioso JC., and, Fink DJ. Long-term neuroprotection achieved with latency-associated promoter-driven herpes simplex virus gene transfer to the peripheral nervous system. Mol Ther. 2005;12:307–313. doi: 10.1016/j.ymthe.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Goss J, Wolfe D, Goins WC, Huang S, Glorioso JC, et al. Protective effect of herpes simplex virus-mediated neurotrophin gene transfer in cisplatin neuropathy. Brain. 2004;127:929–939. doi: 10.1093/brain/awh103. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Wolfe D, Huang S, Goss J, Glorioso JC, Mata M, et al. In vivo gene therapy for pyridoxine-induced neuropathy by herpes simplex virus-mediated gene transfer of neurotrophin-3. Ann Neurol. 2002;51:19–27. doi: 10.1002/ana.10061. [DOI] [PubMed] [Google Scholar]
- Gossen M., and, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Müller G, Hillen W., and, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Yao F, Svensjö T, Winkler T, Lu M, Eriksson C., and, Eriksson E. Tetracycline repressor, tetR, rather than the tetR-mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum Gene Ther. 1998;9:1939–1950. doi: 10.1089/hum.1998.9.13-1939. [DOI] [PubMed] [Google Scholar]
- Pollock R., and, Clackson T. Dimerizer-regulated gene expression. Curr Opin Biotechnol. 2002;13:459–467. doi: 10.1016/s0958-1669(02)00373-7. [DOI] [PubMed] [Google Scholar]
- Wang S, Petravicz J., and, Breakefield XO. Single HSV-amplicon vector mediates drug-induced gene expression via dimerizer system. Mol Ther. 2003;7:790–800. doi: 10.1016/s1525-0016(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Goverdhana S, Puntel M, Xiong W, Zirger JM, Barcia C, Curtin JF, et al. Regulatable gene expression systems for gene therapy applications: progress and future challenges. Mol Ther. 2005;12:189–211. doi: 10.1016/j.ymthe.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, Candolfi M, Puntel M, Xiong W, Muhammad AK, Kroeger K, et al. Regulated expression of adenoviral vectors-based gene therapies: therapeutic expression of toxins and immune-modulators. Methods Mol Biol. 2008;434:239–266. doi: 10.1007/978-1-60327-248-3_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher AR, Schönig K, Brown N, Bujard H., and, Witzgall R. Use of the tetracycline system for inducible protein synthesis in the kidney. J Am Soc Nephrol. 2003;14:2042–2051. doi: 10.1097/01.asn.0000079615.38843.4a. [DOI] [PubMed] [Google Scholar]
- Wang J, Voutetakis A, Zheng C., and, Baum BJ. Rapamycin control of exocrine protein levels in saliva after adenoviral vector-mediated gene transfer. Gene Ther. 2004;11:729–733. doi: 10.1038/sj.gt.3302225. [DOI] [PubMed] [Google Scholar]
- Giménez E, Lavado A, Giraldo P, Cozar P, Jeffery G., and, Montoliu L. A transgenic mouse model with inducible Tyrosinase gene expression using the tetracycline (Tet-on) system allows regulated rescue of abnormal chiasmatic projections found in albinism. Pigment Cell Res. 2004;17:363–370. doi: 10.1111/j.1600-0749.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- Smith JR, Verwaerde C, Rolling F, Naud MC, Delanoye A, Thillaye-Goldenberg B, et al. Tetracycline-inducible viral interleukin-10 intraocular gene transfer, using adeno-associated virus in experimental autoimmune uveoretinitis. Hum Gene Ther. 2005;16:1037–1046. doi: 10.1089/hum.2005.16.1037. [DOI] [PubMed] [Google Scholar]
- Rentsch M, Kienle K, Mueller T, Vogel M, Jauch KW, Püllmann K, et al. Adenoviral bcl-2 transfer improves survival and early graft function after ischemia and reperfusion in rat liver transplantation. Transplantation. 2005;80:1461–1467. doi: 10.1097/01.tp.0000178293.65311.8b. [DOI] [PubMed] [Google Scholar]
- Xiong W, Goverdhana S, Sciascia SA, Candolfi M, Zirger JM, Barcia C, et al. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger K, Le Meur G, Lasne F, Weber M, Deschamps JY, Nivard D, et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol Ther. 2006;13:967–975. doi: 10.1016/j.ymthe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Cawthorne C, Swindell R, Stratford IJ, Dive C., and, Welman A. Comparison of doxycycline delivery methods for Tet-inducible gene expression in a subcutaneous xenograft model. J Biomol Tech. 2007;18:120–123. [PMC free article] [PubMed] [Google Scholar]
- Keswani SC, Buldanlioglu U, Fischer A, Reed N, Polley M, Liang H, et al. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann Neurol. 2004;56:815–826. doi: 10.1002/ana.20285. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M., and, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-κB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Schmidt RE, Green KG, Feng D, Dorsey DA, Parvin CA, Lee JM, et al. Erythropoietin and its carbamylated derivative prevent the development of experimental diabetic autonomic neuropathy in STZ-induced diabetic NOD-SCID mice. Exp Neurol. 2008;209:161–170. doi: 10.1016/j.expneurol.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger K, Mendes-Madeira A, Meur GL, Weber M, Deschamps JY, Nivard D, et al. Oral administration of doxycycline allows tight control of transgene expression: a key step towards gene therapy of retinal diseases. Gene Ther. 2007;14:1668–1673. doi: 10.1038/sj.gt.3303034. [DOI] [PubMed] [Google Scholar]
- McGee Sanftner LH, Rendahl KG, Quiroz D, Coyne M, Ladner M, Manning WC, et al. Recombinant AAV-mediated delivery of a tet-inducible reporter gene to the rat retina. Mol Ther. 2001;3:688–696. doi: 10.1006/mthe.2001.0308. [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, et al. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KA, Lentz SI, Roberts JL., Jr, and, Feldman EL. Criteria for creating and assessing mouse models of diabetic neuropathy. Curr Drug Targets. 2008;9:3–13. doi: 10.2174/138945008783431763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris DS., and, Harrington JE. Herpes simplex virus variants restraint to high concentrations of acyclovir exist in clinical isolates. Antimicrob Agents Chemother. 1982;22:71–77. doi: 10.1128/aac.22.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Zhou Z, Hu J, Fink DJ., and, Mata M. Soluble Nogo receptor down-regulates expression of neuronal Nogo-A to enhance axonal regeneration. J Biol Chem. 2010;285:2783–2795. doi: 10.1074/jbc.M109.046425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M, Mata M, Goss J, Wolfe D, Huang S, Glorioso JC, et al. Prolonged preservation of nerve function in diabetic neuropathy in mice by herpes simplex virus-mediated gene transfer. Diabetologia. 2007;50:1550–1558. doi: 10.1007/s00125-007-0702-4. [DOI] [PubMed] [Google Scholar]