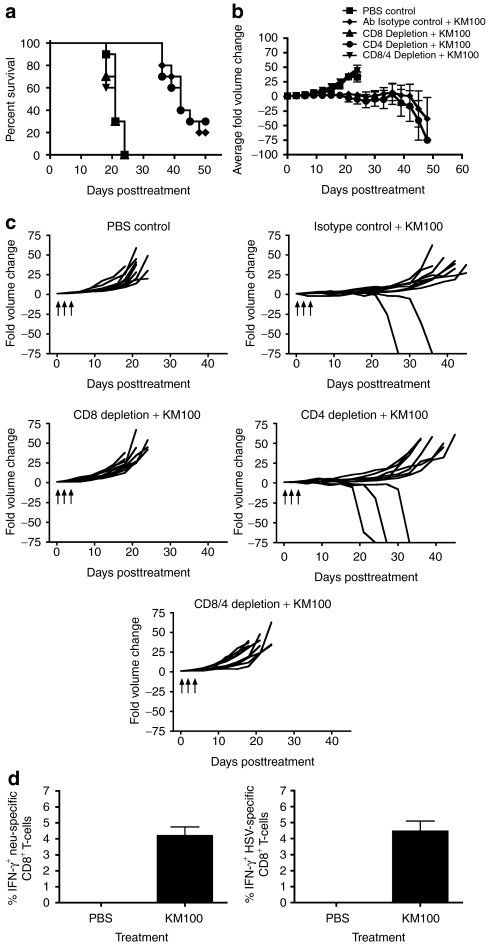
Figure 3.
The in vivo antitumor properties of KM100 are dependent on CD8+ T-cells in the HER-2/neu breast cancer model. Individual components of the T-cell compartment were immunodepleted in HER-2/neu tumor-bearing wild-type mice as in Materials and Methods, and three doses of 2 × 107 pfu of KM100 were administered intratumorally as in Figure 2. (a) Kaplan–Meier survival analysis of T-cell-depleted animals, isotype control antibody (rat IgG) and phosphate-buffered saline (PBS) administered mice following KM100 treatment. Statistical significance was determined by the log-rank test, where P < 0.05. (b) Intergroup comparisons of the average fold changes in HER-2/neu tumor volume within each KM100 or PBS control group. Statistical significance was determined by the Koziol distribution free test when P < 0.001.33 (c) Intragroup comparisons of the fold changes in HER-2/neu tumor volume measurements between the control and KM100 treatment groups. Error bars indicate SE of experimental means within treatment groups from a and b. Arrows indicate time of injection. Antibody-only administration had no significant effects on fold changes in tumor volume or survival. (d) Splenocytes from HER-2/neu tumor-bearing wt mice treated 8 days earlier with KM100 were restimulated ex vivo with the H2-Dq-restricted CD8+ T-cell immunodominant epitope peptides of neu (left panel), Herpes simplex virus (HSV) lysate (right panel) or an irrelevant peptide (LCMV gp-1; data not shown), and HER-2/neu- and HSV-specific CD8+ T-cells were enumerated by intracellular IFN-γ staining. Error bars represent the SE of experimental means. Data were generated from 10 mice per treatment group, combined from two independent experiments.