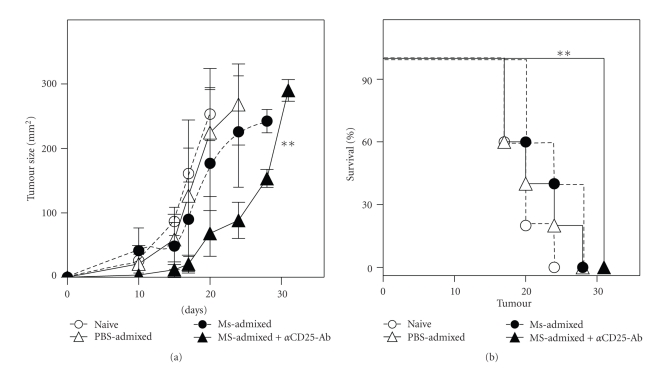
Figure 7.
Therapeutic tumor protection by the dual melanoma antigen-archaeosome vaccine. Mice were injected with 105 B16 melanoma cells subcutaneously on day 0. Vaccination with 30 μg of each peptide in PBS (PBS-admixed) or as mixture of Ms-Gp100 and Ms-TRP (Ms-admixed) was carried out on days 1 and 21 posttumor challenge. One group of mice received the anti-CD25 antibody injection (100 μg), intraperitoneally on day 1 posttumor challenge. Mean tumor size ± SD (n = 5/group) over time is indicated for all groups (a). Animals that received the Ms-admixed vaccine and anti-CD25 antibody showed significantly slower tumor progression over time based on one-way ANOVA Bonferronis Post-test compared to the naïve group (P < .01). Tumor survival (b) is based on animals reaching a maximum tumor size of 300 mm2. Survival for the Ms-Admixed plus anti-CD25 antibody group was also significantly longer (P < .01) relative to the naïve group (n = 5 mice/group) based on Log rank test. The admixed group contained 2.2 mg of lipid and 60 μg of peptide (30 μg of each peptide).