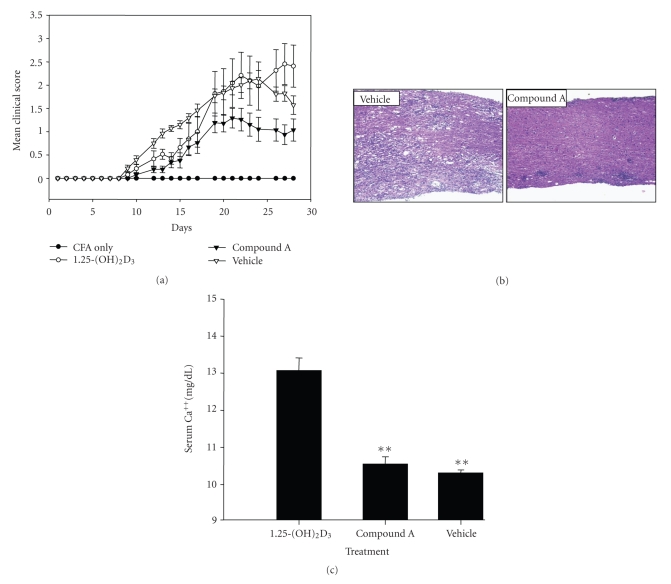
Figure 8.
Oral administration of VDRM is therapeutically efficacious in MOG-induced EAE. (a) Clinical course of EAE after treatment with VDR ligands. MOG immunized C57B6 mice were orally administered with vehicle, 1,25-(OH)2D3 (0.5 μg/kg/d), or compound A (10 μg/kg/d) for 21 days. Each point represents the mean clinical score for particular day in vehicle or VDR ligand-treated (n = 15) groups. Error bars represent mean ± SE. Vehicle group consisted of MOG-immunized mice treated with vehicle (sesame seed oil). CFA group was mock immunized with CFA only (without MOG peptide) and was not treated with any ligands. There was statistically significant reduction of overall EAE disease score between the compound A-treated group and vehicle group and between compound A-treated and 1,25-(OH)2D3-treated group (P < .001) whereas the difference between 1,25-(OH)2D3-treated group and vehicle group is nonsignificant. (b) Compound A improves spinal cord pathology in EAE. On day 28, mice spinal cords were harvested and subjected to histological analysis. Spinal cord sections of vehicle and compound A-treated MOG-immunized mice were analyzed for demyelination by eosin-hematoxylin staining. (c) Compound A does not cause hypercalcemia at therapeutically efficacious dose. At the end of the EAE study, blood ionized calcium was measured 6 hours after the last dose. Value shown represents mean values ± SD of 15, 12, and 6 individual mice of vehicle group, compound A group, and 1,25-(OH)2D3-treated group, respectively; **P < .01.