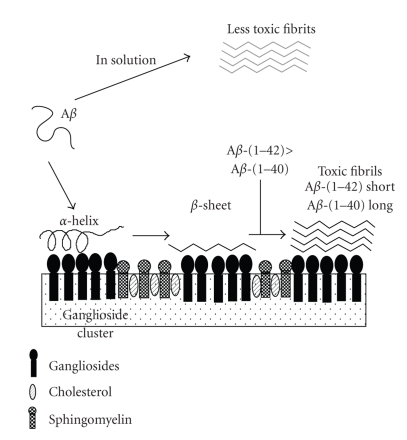
Figure 5.
A model for the formation of toxic amyloid fibrils by amyloid β-protein on ganglioside clusters. Aβ is essentially soluble, and takes an unordered structure in solution. Once ganglioside clusters are generated, Aβ binds to the clusters, forming an α-helix-rich structure at lower protein-to-ganglioside ratios whereas the protein changes its conformation to a β-sheet at higher ratios. The β-sheet form facilitates the fibrillization of Aβ, leading to cytotoxicity. The amyloidogenic activity of Aβ-(1–42) is more than 10-fold that of Aβ-(1–40). Amyloid fibrils formed in solution are much less toxic.