Abstract
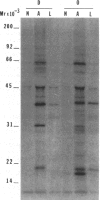
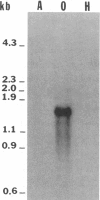
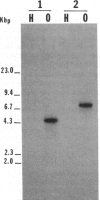
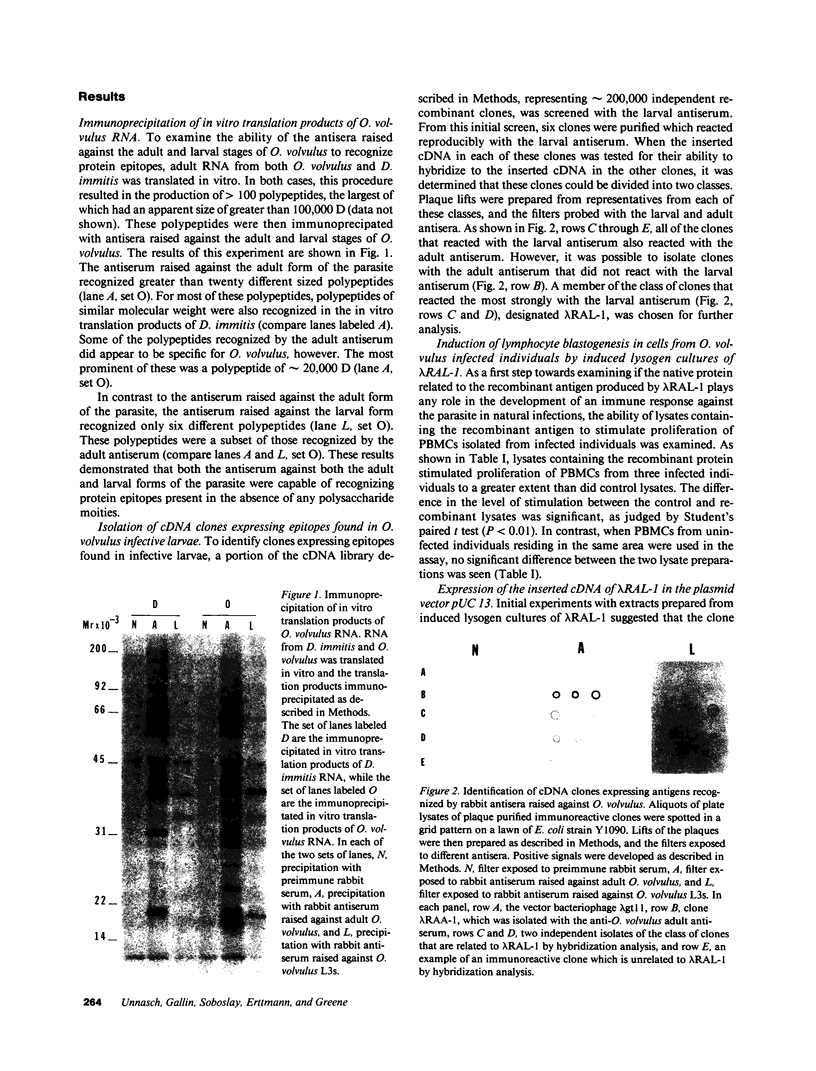
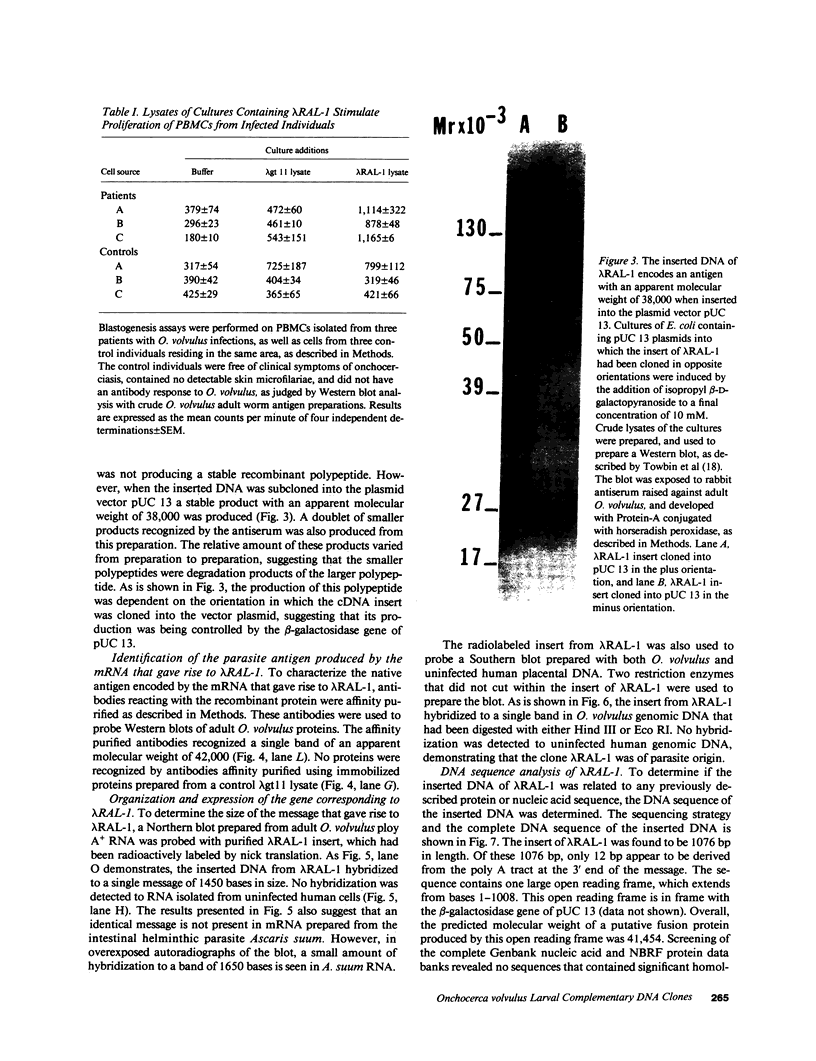
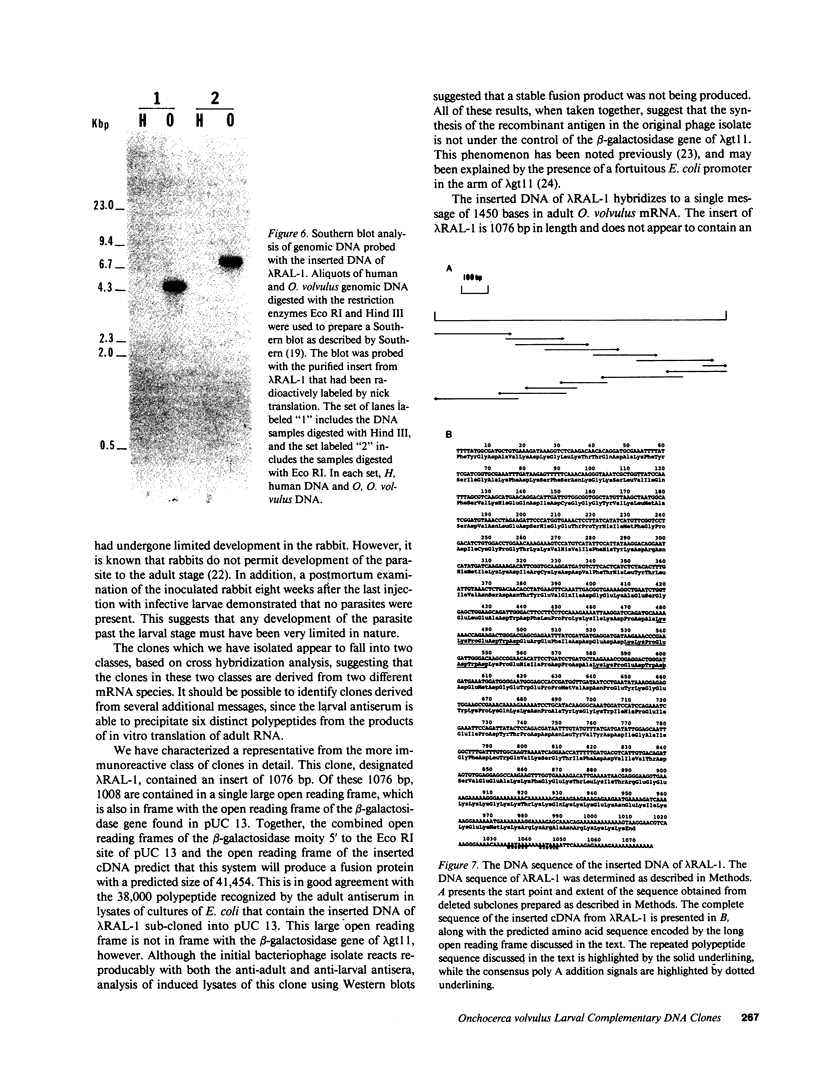
The isolation of recombinant cDNA clones expressing antigens found in Onchocerca volvulus infective larvae is described. To isolate such clones, an expression cDNA library constructed from adult O. volvulus RNA was screened with antiserum raised against infective larvae. One clone, designated lambda RAL-1 was characterized further. The recombinant antigen produced by lambda RAL-1 stimulates proliferation of peripheral blood mononuclear cells from O. volvulus infected humans. Lambda RAL-1 is derived from a 1450 bases message that encodes a protein with an apparent molecular weight of 42,000 in adult O. volvulus. The inserted DNA of lambda RAL-1 contains an open reading frame of 1008 bp. The amino acid sequence predicted by this open reading frame contains three repeats of the sequence KKPEDWD. The identification of clones such as lambda RAL-1 will provide quantities of purified antigens sufficient to begin to study the immune response to and explore the development of immunity against the infectious form of the parasite.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambroise-Thomas P. Immunological diagnosis of human filariases: present possibilities, difficulties and limitations. Acta Trop. 1974;31(2):108–128. [PubMed] [Google Scholar]
- Ballou W. R., Rothbard J., Wirtz R. A., Gordon D. M., Williams J. S., Gore R. W., Schneider I., Hollingdale M. R., Beaudoin R. L., Maloy W. L. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985 May 24;228(4702):996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Intrinsic and extrinsic factors in protein antigenic structure. Science. 1985 Sep 6;229(4717):932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- Bobek L., Rekosh D. M., van Keulen H., LoVerde P. T. Characterization of a female-specific cDNA derived from a developmentally regulated mRNA in the human blood fluke Schistosoma mansoni. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5544–5548. doi: 10.1073/pnas.83.15.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirala S. S. The nucleotide sequence of the lac operon and phage junction in lambda gt11. Nucleic Acids Res. 1986 Jul 25;14(14):5935–5935. doi: 10.1093/nar/14.14.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins W. E., Anders R. F., Pappaioanou M., Campbell G. H., Brown G. V., Kemp D. J., Coppel R. L., Skinner J. C., Andrysiak P. M., Favaloro J. M. Immunization of Aotus monkeys with recombinant proteins of an erythrocyte surface antigen of Plasmodium falciparum. Nature. 1986 Sep 18;323(6085):259–262. doi: 10.1038/323259a0. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Donnelly J. J., Rockey J. H., Bianco A. E., Soulsby E. J. Ocular immunopathologic findings of experimental onchocerciasis. Arch Ophthalmol. 1984 Apr;102(4):628–634. doi: 10.1001/archopht.1984.01040030500036. [DOI] [PubMed] [Google Scholar]
- Donnelly J. J., Taylor H. R., Young E., Khatami M., Lok J. B., Rockey J. H. Experimental ocular onchocerciasis in cynomolgus monkeys. Invest Ophthalmol Vis Sci. 1986 Apr;27(4):492–499. [PubMed] [Google Scholar]
- Enea V., Ellis J., Zavala F., Arnot D. E., Asavanich A., Masuda A., Quakyi I., Nussenzweig R. S. DNA cloning of Plasmodium falciparum circumsporozoite gene: amino acid sequence of repetitive epitope. Science. 1984 Aug 10;225(4662):628–630. doi: 10.1126/science.6204384. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Greene B. M., Fanning M. M., Ellner J. J. Non-specific suppression of antigen-induced lymphocyte blastogenesis in Onchocerca volvulus infection in man. Clin Exp Immunol. 1983 May;52(2):259–265. [PMC free article] [PubMed] [Google Scholar]
- Greene B. M. Primate model for onchocerciasis research. Ciba Found Symp. 1987;127:236–243. doi: 10.1002/9780470513446.ch16. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Ham P. J., Banya A. J. The effect of experimental Onchocerca infections on the fecundity and oviposition of laboratory reared Simulium sp. (Diptera, Simuliidae). Tropenmed Parasitol. 1984 Mar;35(1):61–66. [PubMed] [Google Scholar]
- Higashi G. I. Immunodiagnostic tests for protozoan and helminthic infections. Diagn Immunol. 1984;2(1):2–18. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood B., Smith P., Marshall T., Prost A. Relationships between mortality, visual acuity and microfilarial load in the area of the Onchocerciasis Control Programme. Trans R Soc Trop Med Hyg. 1983;77(6):862–868. doi: 10.1016/0035-9203(83)90308-5. [DOI] [PubMed] [Google Scholar]
- Kozek W. J., Figueroa Marroquin H. Attempts to establish Onchocerca volvulus infection in primates and small laboratory animals. Acta Trop. 1982 Dec;39(4):317–324. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lal R. B., Lynch T. J., Nutman T. B. Brugia malayi antigens associated with lymphocyte activation in filariasis. J Immunol. 1987 Sep 1;139(5):1652–1657. [PubMed] [Google Scholar]
- Mackenzie C. D., Williams J. F., Sisley B. M., Steward M. W., O'Day J. Variations in host responses and the pathogenesis of human onchocerciasis. Rev Infect Dis. 1985 Nov-Dec;7(6):802–808. doi: 10.1093/clinids/7.6.802. [DOI] [PubMed] [Google Scholar]
- Ngu J. L. Immunological studies on onchocerciasis. Varying skin hypersensitivity and leucocyte migration inhibition in a clinical spectrum of the disease. Acta Trop. 1978 Sep;35(3):269–279. [PubMed] [Google Scholar]
- Nogami S., Hayashi Y., Tanaka M., Korenaga M., Tada I., Tanaka H. Antigenic similarity of Onchocerca volvulus to other helminths examined by monoclonal antibodies against O. volvulus. Jpn J Exp Med. 1986 Aug;56(4):177–183. [PubMed] [Google Scholar]
- Ravetch J. V., Kochan J., Perkins M. Isolation of the gene for a glycophorin-binding protein implicated in erythrocyte invasion by a malaria parasite. Science. 1985 Mar 29;227(4694):1593–1597. doi: 10.1126/science.3883491. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier P. H., Cortese R. A fast and simple method for sequencing DNA cloned in the single-stranded bacteriophage M13. J Mol Biol. 1979 Mar 25;129(1):169–172. doi: 10.1016/0022-2836(79)90068-8. [DOI] [PubMed] [Google Scholar]
- Schulz-Key H., Albiez E. J., Büttner D. W. Isolation of living adult Onchocerca volvulus from nodules. Tropenmed Parasitol. 1977 Dec;28(4):428–430. [PubMed] [Google Scholar]
- Shah J. S., Karam M., Piessens W. F., Wirth D. F. Characterization of an Onchocerca-specific DNA clone from Onchocerca volvulus. Am J Trop Med Hyg. 1987 Sep;37(2):376–384. doi: 10.4269/ajtmh.1987.37.376. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Wong M. M., Guest M. F., Lavoipierre M. J. Dirofilaria immitis: fate and immunogenicity of irradiated infective stage larvae in beagles. Exp Parasitol. 1974 Jun;35(3):465–474. doi: 10.1016/0014-4894(74)90052-6. [DOI] [PubMed] [Google Scholar]
- Yates J. A., Higashi G. I. Brugia malayi: vaccination of jirds with 60cobalt-attenuated infective stage larvae protects against homologous challenge. Am J Trop Med Hyg. 1985 Nov;34(6):1132–1137. doi: 10.4269/ajtmh.1985.34.1132. [DOI] [PubMed] [Google Scholar]