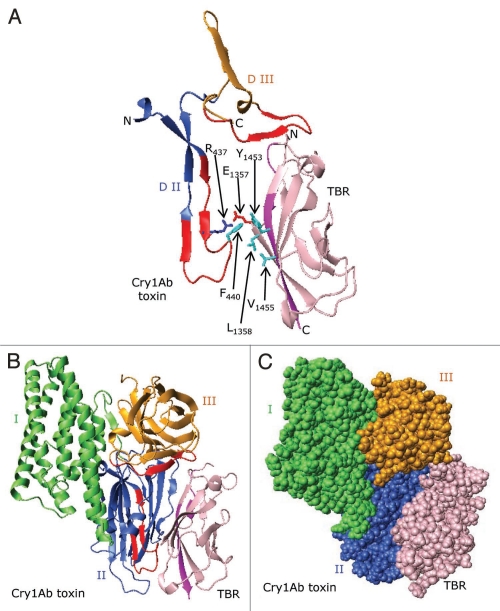
Figure 9.
A proposed model showing docking of the Cry1Ab toxin to BT-R1. (A) Ribbon structures showing docking of the putative receptor-binding sequence of the Cry1Ab toxin (residues 422–498) to the TBR (residues 1349–1460) in BT-R1. The structure for the TBR was generated from the X-ray crystal structures of homologous cadherin domains as described in the legend to Figure 6. Note that in the interface between the Cry1Ab toxin and the TBR of BT-R1 there are potential hydrophobic interactions involving surface-exposed Phe440 in Cry1Ab and Leu1358, Tyr1453 and Val1455 in the TBR of BT-R1. Also, there is probable electrostatic interaction between the positively charged Arg437 in Cry1Ab and the negatively charged Glu1357 in the TBR. Glu1357, Leu1358, Tyr1453 and Val1455 lie either within or immediately adjacent to the signature sequences in the TBR of BT-R1 (Fig. 6 and blue arrows). Ribbon (B) and space-filling (C) representations showing docking of Cry1Ab toxin to TBR. Domains I, II and III of the toxin are colored green, blue and gold, respectively. The TBR is colored pink. Surface-exposed residues 432–449 and 480–493 in Cry1Ab are colored red, and residues 1349–1354 and 1451–1460 (signature sequences) in the TBR are colored purple.