Abstract
Locusts possess an identified neuron, the descending contralateral movement detector (DCMD), conveying visual information about impending collision from the brain to thoracic motor centers. We built a telemetry system to simultaneously record, in freely behaving animals, the activity of the DCMD and of motoneurons involved in jump execution. Co-contraction of antagonistic leg muscles, a required preparatory phase, was triggered after the DCMD firing rate crossed a threshold. Thereafter, the number of DCMD spikes predicted precisely motoneuron activity and jump occurrence. Additionally, the time of DCMD peak firing rate predicted that of jump. Ablation experiments suggest that the DCMD, together with a nearly identical ipsilateral descending neuron, is responsible for the timely execution of the escape. Thus, three distinct features that are multiplexed in a single neuron’s sensory response to impending collision – firing rate threshold, peak firing time, and spike count – likely control three distinct motor aspects of escape behaviors.
Introduction
The transformation of sensory signals into motor commands plays a pivotal role in the generation of behavior. Much work, both in vertebrates and invertebrates, has focused on characterizing how the spike trains of sensory neurons may determine the motor output of an organism (Mountcastle et al. 1975; Newsome et al., 1988; Trimarchi and Schneiderman, 1993; Lewis and Kristan, 1998; Edwards et al., 1999; van Hateren et al., 2005; Santer et al., 2006; Marsat and Pollack 2006; Lima and Miesenböck 2005; Korn and Faber, 2005; Ishikane et al., 2005; De Lafuente and Romo, 2005; Gu et al., 2008; Cohen and Newsome, 2009; Nienborg and Cummings, 2009). In particular, both the mean number of spikes, and firing rate thresholds in sensory neuron populations have been implicated (Camhi and Levy, 1989; Cook and Maunsell, 2002; Roitman and Shadlen, 2002). Yet, little is known about how the time-varying firing rate of sensory neurons control the specific motor sequences underlying ongoing, complex motor behaviors.
Collision avoidance and escape behaviors provide a favorable model to study this question. They are critical for survival and are implemented by specialized neural circuits in several species (Wang and Frost, 1992; Graziano et al., 1994; Wicklein and Strausfeld, 2000; Yamamoto et al., 2003; Preuss et al., 2006; Olivia et al., 2007; Fotowat et al., 2009). In locusts, the third neuropil in each of the two optic lobes contains an identified neuron, the lobula giant movement detector (LGMD) that responds specifically to objects approaching on a collision course in its associated visual hemifield, or their two-dimensional projection: looming stimuli (Hatsopoulos et al., 1995; Schlotterer, 1977; Rind and Simmons, 1992; Judge and Rind, 1997; Peron and Gabbiani, 2009). Each LGMD synapses in the brain onto the descending contralateral movement detector (DCMD) neuron, such that their spikes are in one-to-one correspondence (Rind, 1984; Killmann and Schurmann, 1985). In response to looming stimuli the firing rate of these neurons gradually increases, peaks, and rapidly decreases before expected collision (Gabbiani et al., 1999). Similar response profiles have now been described in neurons of wide-ranging species (pigeon: Sun and Frost, 1998; frog: Kang and Nakagawa, 2010; fish: Preuss et al., 2006; fruit fly: Fotowat et al., 2009). In locusts, this response profile is robust to a broad spectrum of stimulus changes, suggesting that it may play an important role in the generation of escape behaviors (Gabbiani et al., 2001).
From the brain, each DCMD axon projects through the contralateral nerve cord to motor centers involved in jump and flight steering (O Shea et al. 1974; Simmons, 1980). In particular, the DCMDs make both direct and indirect synaptic contacts with the Fast Extensor Tibia (FETi) motoneuron of the hind leg, and indirect connections to the flexor tibia motoneurons (Burrows and Rowell, 1973; Pearson et al., 1980; Pearson and Robertson, 1981).
The involvement of DCMD activity in jump escape behaviors has been studied, but its role remains unclear (Fotowat and Gabbiani, 2007; Burrows, 1996; Santer et al., 2005). Up to now, it was impossible to record simultaneously from the DCMD and motoneurons during freely executed, visually guided jump escape behaviors. Consequently, it was not possible to observe how sensory and motor activities are related on a trial-by-trial basis. To achieve this goal, we built a telemetry system capable of wireless transmission of neural and muscle recordings. This system was sufficiently small that locusts could carry it as a backpack and still respond to looming stimuli by jumping. We also developed a technique allowing us to selectively laser ablate the DCMD before behavioral jump experiments to further assess the relationship between its neural activity and escape behaviors.
Results
Our digital telemetry system allowed us to monitor simultaneously the sensory and motor activity evoked by looming stimuli during collision avoidance behaviors (Methods, Supp. Fig. 1). The simulated objects were black discs on a bright background with various size to speed ratios, l/|v|, where l is the disc radius and |v| the approach speed. This parameter has units of time and determines the stimulus angular size, θ(t), since by trigonometry the tangent of θ/2 is the ratio of l to the object’s distance (v × t; Fig. 1, Methods). Equivalently, l/|v| is the time remaining to collision when the stimulus subtends 90° on the retina. Thus, the faster the stimulus approach speed, |v|, the smaller l/|v|. Looming stimuli were always presented on one side of the animal so that a single DCMD neuron was stimulated.
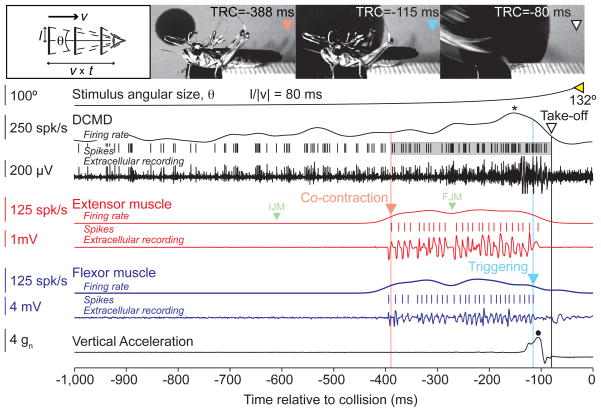
Figure 1.
Neural, muscle, and acceleration recordings obtained during jump behavior using wireless telemetry (see also Supp. Movie 1). Time markers and corresponding video frames for the onset of co-contraction, its end (triggering), and take-off are indicated with  ,
,  , and ▽, respectively;
, and ▽, respectively;  marks the final angular size. The timing of the Initial and Final Joint Movements (IJM and FJM) are marked by the symbols [
marks the final angular size. The timing of the Initial and Final Joint Movements (IJM and FJM) are marked by the symbols [ ] (see Results). Co-contraction starts before, and take-off occurs after the peak (*) DCMD firing rate (TRC= Time Relative to Collision). The shaded area around the DCMD spikes corresponds to the time period over which they were counted for further analysis (see Results). The right and left bounds of the shaded area are the co-contraction onset and take-off time, respectively. Peak vertical acceleration marked by a •. Top left inset: Schematics of the stimuli. Discs of radius l approaching at constant speed v subtend an angle θ at the retina. By convention v<0 for approaching objects and t<0 before collision (bottom axis). (v × t) is the distance of the object to the eye.
] (see Results). Co-contraction starts before, and take-off occurs after the peak (*) DCMD firing rate (TRC= Time Relative to Collision). The shaded area around the DCMD spikes corresponds to the time period over which they were counted for further analysis (see Results). The right and left bounds of the shaded area are the co-contraction onset and take-off time, respectively. Peak vertical acceleration marked by a •. Top left inset: Schematics of the stimuli. Discs of radius l approaching at constant speed v subtend an angle θ at the retina. By convention v<0 for approaching objects and t<0 before collision (bottom axis). (v × t) is the distance of the object to the eye.
Energy storage starts before, and take-off occurs after peak DCMD firing rate
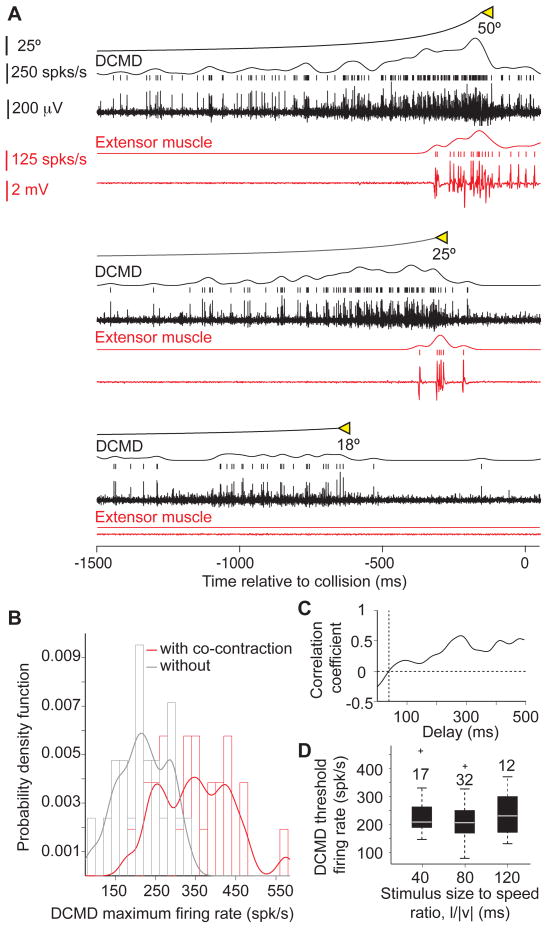
Fig. 1 shows a trial in which a locust jumped in response to a looming stimulus (Supp. Movie 1). Spikes from the DCMD, the Fast Extensor Tibiae (FETi) and flexor motoneurons were obtained by extracellular recording from the contralateral nerve cord, the hind leg extensor and flexor muscles, respectively. The time course of vertical acceleration was measured by an on-board accelerometer. The locust jump is a complex behavior, consisting of several distinct phases, during which the animal orients itself away from the approaching object using its middle legs and stores the energy required for take-off in the elastic elements of its hind legs (Burrows, 1996; Santer et al., 2005). By monitoring the position of the hind leg femur-tibia joint, we previously showed that after an initial flexion of the tibia, the joint moves to align the leg parallel to the body (initial joint movement, IJM; Fotowat and Gabbiani, 2007). Subsequently, the flexor and extensor muscles contract simultaneously to store the mechanical energy required for the jump (co-contraction). This leads to a final femur-tibia joint movement (FJM), which is followed by cessation of activity in the flexors (triggering) that allows energy release and take-off. Looming stimuli with l/|v| values larger than 40 ms led to jumps before the expected collision time. As illustrated in Fig. 1, locusts started to accelerate towards the end of co-contraction, and vertical acceleration peaked immediately after triggering (mean: 5.8 gn, SD: 1.3; number of locusts, nL = 3, number of trials, nT = 20; Methods). During co-contraction the flexors and extensors fired fairly regular spike trains (mean ISI: 14 ms, CV: 0.69, nL = 4, nT = 54), and the number of their spikes were highly correlated (ρ=0.8, p <10−9). The DCMD firing rate gradually increased, peaked, and sharply decreased before projected collision, as observed in fixed animals (Fotowat and Gabbiani, 2007). Fig. 1 shows that the co-contraction phase started before the DCMD firing rate reached its peak (mean: 169 ms, SD: 49, nL = 3, nT = 24), whereas take-off occurred afterward. This was the case in every trial for all animals (Fig. 2A).
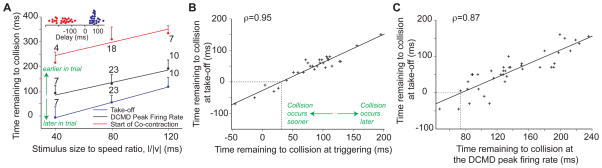
Figure 2.
Relative timing of jump-escape stages in freely behaving animals. A) Timing of co-contraction onset (red), DCMD peak firing rate (black) and take-off (blue) in response to looming stimuli with l/|v|= 40, 80, and 120 ms (mean and SD; nT shown on figure). The timing of these stages was highly correlated with l/|v|, ρ=0.57, 0.69, and 0.78, respectively. Slopes (α) and intercepts (δ) of linear fits were as follows. Start of co-contraction: α=1.33 (SD: 0.37), δ=191 ms (SD: 33); DCMD peak: α=1.26 (SD: 0.22), δ=34 ms (SD: 19); Take-off: α=1.55 (SD: 0.20), δ=−69ms (SD: 18). Top inset: Representative delays between DCMD peak and co-contraction onset (red) and between peak and take-off (blue; nT = 23). Positive delays correspond to events after the peak (data points staggered vertically for clarity). B) The end of co-contraction (triggering) and take-off were highly correlated (ρ = 0.95, data pooled across l/|v| values). Linear fit slope: 0.89 (SD: 0.06); intercept: −27 ms (SD: 3.7), indicating that take-off occurs approximately 27 ms after triggering (dashed line). C) Timing of DCMD peak firing rate and take-off relative to expected collision time were highly correlated (ρ = 0.87, data pooled across l/|v| values). Linear fit slope: 0.94 (SD: 0.09); intercept: −70 ms (SD: 13), indicating that take-off occurs approximately 70 ms after the DCMD peak (dashed line). nL = 9 for DCMD and take-off data, nL = 4 for co-contraction data.
Which aspects of the motor and sensory activity determine the timing of the jump? We found that the time at which the co-contraction ended (triggering) was highly correlated with take-off (ρ = 0.95, p<10−9). Moreover, this correlation exists regardless of l/|v|, since the partial correlation coefficient between these two variables controlling for l/|v| remained high (ρpart = 0.94, p<10−9). On average take-off occurred 36 ms after triggering (SD: 15, nL = 4, nT = 29; Fig. 2B, dashed line) and 90% of the variance in the timing of take-off could be explained by the timing of triggering. At the sensory level, we found that the timing of the DCMD peak firing rate and take-off were highly correlated as well (ρ = 0.87, p<10−9) and that the partial correlation coefficient between these variables controlling for l/|v| also remained high (ρpart = 0.73, p=9.2×10−8). Locusts took off on average 70 ms (SD: 13) after the DCMD firing rate peaked, regardless of l/|v| (Fig. 2C, dashed line) and the timing of the peak accounted for 75% of the variance of the take-off time.
Comparison of sensory-motor activity in trials with and without jump
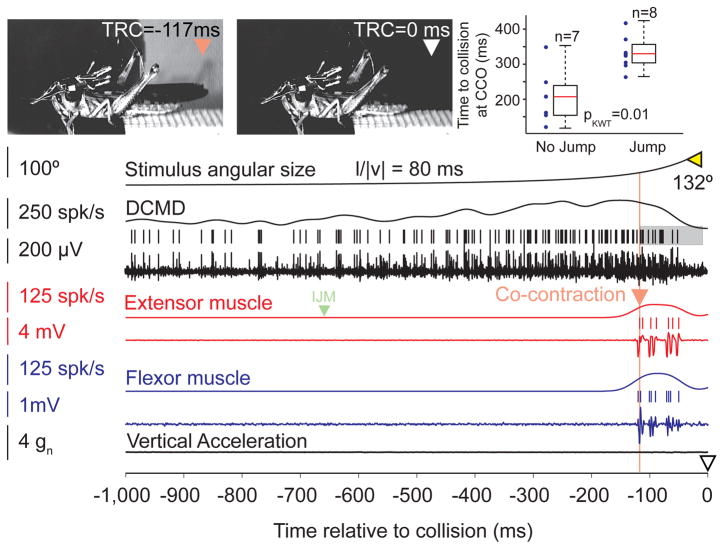
Not all looming stimuli led to a final take-off. Thus, locusts jumped with a median probability of 32%. The jump probability was significantly reduced compared to that of animals without a telemetry backpack (Fotowat and Gabbiani, 2007; median: 64%, pKWT=0.003). Fig. 3 shows a trial in which the same locust as in Fig. 1 did not jump (Supp. Movie 2). It started preparing by co-contracting its hind leg flexor and extensor muscles. However, compared to jump trials, the co-contraction started late, such that after a few spikes in the extensor the looming stimulus reached its full size, the DCMD firing rate declined, and the co-contraction ended. This was the case in 85% of trials without take-off, whereas in the remaining 15% the co-contraction failed to initiate altogether.
Figure 3.
Neural and muscle recordings during a trial in which the animal did not take off (see also Supp. Movie 2). The symbols  and ▽ mark the start of co-contraction, and the expected collision time, respectively (and corresponding video frames);
and ▽ mark the start of co-contraction, and the expected collision time, respectively (and corresponding video frames);  marks the final angular size. The locust prepares to jump by co-contracting its flexor and extensor muscles, but never takes off (same animal as in Fig. 1). The shaded area around the DCMD spikes corresponds to the time period over which they were counted for further analysis (see Results). The right and left bounds are the co-contraction onset and the time at which the DCMD firing rate falls below 5 spk/s respectively. Top right: Co-contraction onset (CCO) occurred significantly earlier for jumps (all trials at l/|v| = 80 ms, same locust as in main panel). Individual trial values shown on left (dots); corresponding box plots on right.
marks the final angular size. The locust prepares to jump by co-contracting its flexor and extensor muscles, but never takes off (same animal as in Fig. 1). The shaded area around the DCMD spikes corresponds to the time period over which they were counted for further analysis (see Results). The right and left bounds are the co-contraction onset and the time at which the DCMD firing rate falls below 5 spk/s respectively. Top right: Co-contraction onset (CCO) occurred significantly earlier for jumps (all trials at l/|v| = 80 ms, same locust as in main panel). Individual trial values shown on left (dots); corresponding box plots on right.
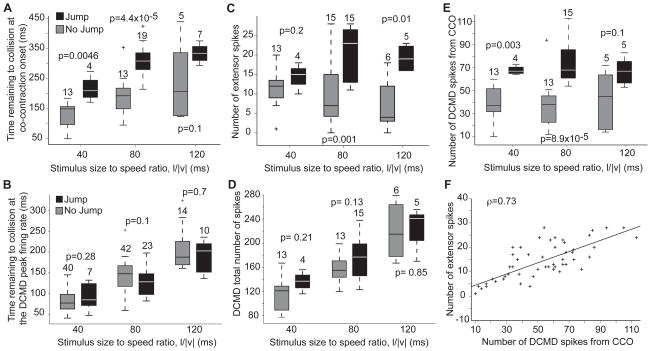
Across animals, we found that co-contraction onset occurred significantly earlier relative to collision in jump trials (Fig. 4A), whereas the timing of the DCMD peak itself did not change (Fig. 4B). Thus, while the DCMD peak time predicts the time of take-off, it fails to predict its occurrence. Since co-contraction started earlier in jump trials, the number of extensor spikes was also significantly higher (Fig. 4C). In contrast, there was no difference in the total number of DCMD spikes between jump and no-jump trials (Fig. 4D), although the peak DCMD firing rate was higher in jump trials (Supp. Fig. 2A). However, we found that if we started counting the DCMD spikes from co-contraction onset rather than stimulus onset (shaded areas in Figs. 1 and 3), their number was significantly higher in jump trials (Fig. 4E). Furthermore, the number of DCMD spikes from co-contraction onset was highly correlated with the number of extensor spikes (ρ = 0.73, p< 10−9, Fig. 4F), such that on average 4.3 DCMD spikes led to one extensor spike (SD: 2.1 spk). To further test for a possible causal relation between the DCMD and extensor firing rates following co-contraction onset, we designed looming stimuli that abruptly stopped in mid-course and resumed their looming immediately thereafter. This often caused the DCMD firing rate to peak twice: once before and once after the abrupt motion cessation (in 13 out of 17 trials, nL = 3). Under these conditions, the firing rate in the extensor faithfully tracked that of the DCMD in 10 of these 13 trials (Supp. Fig. 2B). Of the remaining 3 trials, 2 failed to elicit extensor spikes, while the last one elicited spikes only after the second DCMD peak.
Figure 4.
Comparison between sensory and motor activity in Jump (J) and No Jump (NJ) trials (see also Supp. Fig. S2). A) Co-contraction started earlier in J trials. B) Timing of the DCMD peak rate was not significantly different in J and NJ trials. C) The number of extensor spikes was higher and did not change significantly with l/|v| (pKWT-J = 0.18, pKWT-NJ = 0.15). D) The total number of DCMD spikes was not significantly different in J and NJ trials. E) The number of DCMD spikes from Co-Contraction Onset (CCO) was higher in J trials and did not change significantly with l/|v| (pKWT-J = 0.6, pKWT-NJ = 0.9). F) In both J and NJ trials the number of extensor spikes from CCO was positively correlated with the number of DCMD spikes (linear fit slope: 0.2, SD: 0.09; intercept: 2 spk, SD: 1.5). Kruskal-Wallis test p values and nT shown next to box plots. Data from 4 locusts (except B, where nL = 10).
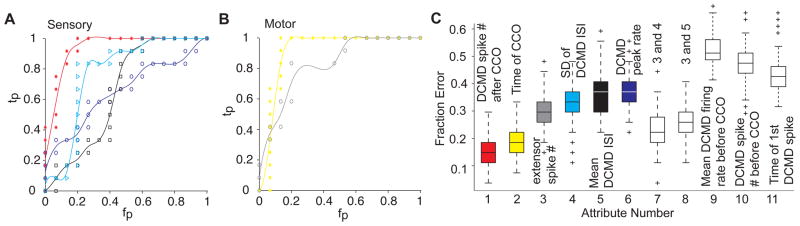
Which motor or sensory attribute best predicts the occurrence of a jump? To address this question we trained a naïve Bayes classifier to discriminate between jump and no-jump trials based on various sensory and motor attributes (Fig. 5). The number of extensor spikes predicted the occurrence of a jump with an accuracy of 70% (SD: 7%). The time of co-contraction onset did even better (83%, SD: 4%). On the sensory side, the number of DCMD spikes after co-contraction onset had a similar accuracy (82%, SD: 6%). In contrast, DCMD attributes computed before co-contraction onset consistently performed poorly. Although several other attributes predicted the occurrence of a jump, none did as well as the time of co-contraction onset or the number of DCMD spikes after co-contraction onset. In particular, the variability of the DCMD spike train, as embodied by the standard deviation of its inter-spike interval (ISI) distribution, could predict a substantial fraction of the jumps, but it did not improve the prediction accuracy given by the number of DCMD spikes after co-contraction onset. On the other hand, adding information about the mean or SD of the DCMD ISI to the number of extensor spikes, significantly improved the performance of the classifier (Fig. 2C, attributes 7 and 8). As we explain in the Supp. Text and Fig. S3, it is therefore likely that the increase in the number of DCMD spikes (and a concurrent decrease in the mean and SD of the ISI) results in better summation of these spikes in the FETi and other thoracic interneurons.
Figure 5.
Predicting take-off from sensory and motor attributes (see also Supp. Fig. S3). A) Receiver Operating Characteristics (ROC) curve for naïve Bayes classifiers trained to distinguish between Jump (J) and No Jump (NJ) trials based on the number of DCMD spikes (red), the SD of the DCMD ISI (cyan), the mean DCMD ISI (black), and the DCMD peak firing rate (blue). Abbreviations: tp= true positives, fp= false positives. B) ROC curve for classifiers trained with the timing of co-contraction onset (CCO, yellow) and the number (#) of extensor spikes (gray). C) Misclassification rate of different classifiers trained and tested with 100 random data shuffles (box plots; chance level: 0.5). Attributes are as follows (including medians in J and NJ trials and difference significance level). 1: Number of DCMD spikes from CCO (J: 67, NJ: 38, pKWT: 9.4×10−8); 2: Time of CCO relative to projected collision (J: 307 ms, NJ: 152, pKWT: 4.4×10−7); 3: Number of extensor spikes (J: 20, NJ: 10, pKWT: 3.2×10−5); 4: SD of DCMD ISI after CCO (J: 2 ms, NJ: 3, pKWT: 0.0141); 5: Mean DCMD ISI after CCO (J: 3 ms, NJ: 4, pKWT: 4.0×10−3); 6: DCMD peak firing rate (J: 427 spk/s, NJ: 362, pKWT: 1.9×10−3); 9: Mean DCMD firing rate before CCO (J: 34 spk/s, NJ: 32, pKWT: 0.74); 10: number of DCMD spikes before CCO (J: 112, NJ: 105, pKWT: 0.15); 11: Time of 1st DCMD spike from stimulus onset (J: 3923 ms, NJ: 3564, pKWT: 0.06).
Co-contraction is triggered a fixed delay after a threshold DCMD firing rate
Both the timing of co-contraction (Fig. 2A), and a threshold in the DCMD firing rate vary linearly with l/|v| (Gabbiani et al., 2002). We therefore investigated whether a threshold in the DCMD firing rate could play a role in triggering the co-contraction using three different approaches. First, we presented locusts with looming stimuli stopping at various final sizes. Stopping the stimulus at smaller final sizes allowed us to reduce excitation to the DCMD before it peaks, and therefore manipulate its maximum firing rate (Gabbiani et al., 2005). Fig. 6A shows the DCMD and extensor muscle activity evoked in response to such stimuli. At the lowest final size no extensor spikes where recorded. Increasingly larger final sizes caused a concurrent increase in the DCMD maximal firing rate and the number of extensor spikes. While final angular size was not always a strong predictor of the occurrence of co-contraction (Supp. Fig. 4A and B), the probability distribution of the DCMD maximum firing rate for trials with co-contraction was shifted to larger firing rates compared to trials without co-contraction (Fig. 6B). Using a linear discriminant, we could predict with an accuracy of 83% the occurrence of co-contraction based on whether the maximum DCMD firing rate exceeded 248 spk/s (Supp. Fig. 4C). Second, in a subset of these trials (nT = 9, nL= 6) only one or two extensor spikes were recorded after the stimulus had stopped and the DCMD had reached its maximum activity (Supp. Fig. 4D). Thus, the maximum DCMD activity in these trials, 300 spk/s on average, was just above the threshold required to trigger the co-contraction (SD: 72). This value is close to that suggested to trigger collision avoidance in flight (Santer et al., 2006) and not significantly higher than that estimated using a linear discriminant (t-test, p = 0.073). Furthermore, in these trials the average delay between the maximum DCMD firing rate and the start co-contraction was 36 ms (SD: 23).
Figure 6.
A DCMD firing rate threshold contributes to triggering co-contraction (see also Supp. Fig. S4). A) Example of neural and muscle recordings in response to looming stimuli with three final angular sizes (from bottom to top: 18, 25 and 50°; l/|v|=80 ms). As the final size increases the DCMD maximum firing rate and the total number of extensor spikes increase as well. Co-contraction did not occur for a final size of 18°. B) Probability Density Function (PDF) for the DCMD maximum firing rate in trials with, and without co-contraction (red and gray, respectively). The PDF is estimated using a non-parametric fit to the firing rate histogram as the sum of Gaussian kernels with bandwidths equal to 20 spk/s. C) Correlation coefficient between DCMD firing rate and l/|v| plotted as a function of delay before co-contraction onset. The correlation coefficient equals zero 40 ms before co-contraction onset. D) At that time the DCMD firing rate does not depend on l/|v| (pKWT = 0.6), and has an average value of 225 spk/s (SD: 73). Box plots of data from 4 locusts presented with full-expansion looming stimuli.
As a third approach for assessing the role of a DCMD firing rate threshold in triggering co-contraction, we carried out a correlation analysis on the data recorded in trials with full stimulus expansion. We hypothesized that if the co-contraction is triggered a fixed delay after a threshold DCMD firing rate is reached, the value of the firing rate at that delay must be independent of l/|v|. Consistent with this hypothesis, the DCMD firing rate and the stimulus size to speed ratio were uncorrelated 40 ms prior to co-contraction onset (Fig. 6C). The firing rate at this delay did not significantly change with l/|v| (pKWT=0.6) and had an average of 225 spk/s (SD: 73; Fig. 6D), close to the values predicted by the two other methods considered above. Taking into account the observed variability, we conclude that the co-contraction is triggered approximately 40 ms after the DCMD approximately exceeds a firing rate of 250 spk/s.
Using data from the same experiments, we next checked that the total number of DCMD spikes from trial start to co-contraction onset was only weakly correlated with the time of co-contraction (ρ = 0.07, p = 0.6). This result is also consistent with a change in DCMD firing rate immediately before co-contraction onset, such as a firing rate threshold, being more critical than accumulation of spikes over the entire trial. The trial-by-trial correlation of the firing rate threshold time with that of co-contraction onset was high (ρ = 0.6, p < 10−9; Supp. Fig. 4E) and predicted 36% of the variance of co-contraction onset. Furthermore, this correlation value decreased by 1/3 when we randomly shuffled these two variables across trials (ρ = 0.39, p = 0.01; mean over 100 shuffles, SD: 0.07) and was significantly smaller than that obtained without shuffling (p = 0.001, z-test). These results also suggest that a DCMD firing rate threshold plays a trial-by-trial role in determining the onset of co-contraction, but that other neurons may contribute as well.
To quantify the steepness of the threshold, we plotted the extensor firing rate as a function of the DCMD firing rate and computed the DCMD firing rate change resulting in the extensor sweeping from 5 to 25% of its peak rate (Supp. Fig. 4F). On average the corresponding relative DCMD firing rate change amounted to ~5% and was thus approximately 4 times steeper than that of the extensor (20%).
Is the DCMD activity necessary for looming-evoked escape behaviors?
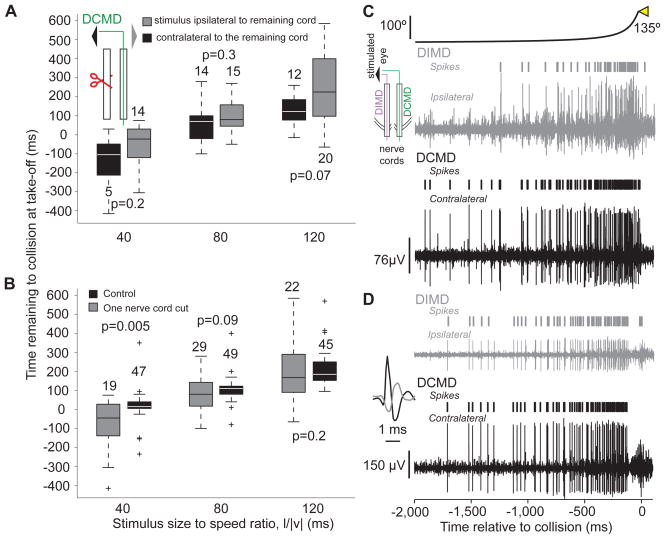
So far, the results suggest that the DCMD strongly contributes to the execution of various phases of looming-evoked escape behaviors. We next asked: Is the DCMD activity necessary for their generation? To address this question, we sectioned one of the two nerve cords (nL=6), and presented looming stimuli to the eye ipsi- or contralateral to the intact nerve cord. We compared the timing and probability of take-off before and after this procedure. We found that, irrespective of the stimulated eye, these locusts still took off and that the timing of take-off remained as positively correlated with l/|v| as in control experiments (ρ = 0.9, p <10−9). Moreover, the take-off time was not significantly different when the stimulus was presented to the eye ipsi- or contralateral to the remaining nerve cord (Fig. 7A) and was significantly delayed only for l/|v|=40 ms (Fig. 7B; a similar result was obtained at l/|v| = 30 ms, data not shown). The variability in the take-off time was however increased, as reported previously for the time of the initial flexion in tethered locusts (Santer et al., 2008). Additionally, the probability of take-off was reduced on average by 51% (SD: 24%) for stimulation of the eye ipsilateral to the intact cord and 64% (SD: 27%) for stimulation of the contralateral eye. These reductions were not significantly different from each other (pKWT=0.42).
Figure 7.
Locusts with one nerve cord sectioned still jump in response to looming stimuli and comparison between looming evoked activities in the DCMD and DIMD (see also Supp. Fig. S5). A) In animals with one sectioned nerve cord, no significant difference in the timing of take-off was observed, irrespective of the stimulated eye (box plots; nL = 6). Inset: stimulated eye and sectioning procedure (red scissors). B) The timing of take-off was significantly delayed at l/|v|=40 ms relative to control. The timing of take-off showed higher variability after cutting one nerve cord. Box plots of data pooled across trials where the stimulus was presented to the eye ipsi- and contralateral to the remaining nerve cord, since those take-off times did not show a significant difference (A). C) Looming-evoked activities in the DCMD and DIMD obtained by simultaneous recording from both nerve cords (inset). In this locust, the DCMD and DIMD spikes were not always coincident. D) Recording from a different locust in which the DCMD and DIMD spikes were coincident. Inset: example of coincident DIMD (gray) and DCMD spikes on an expanded time scale. The ipsi- and contralateral extracellular recordings are plotted on the same vertical scale.
Since locusts with a nerve cord sectioned contralateral to the stimulated eye jump at the same time as control animals, there must exist at least one looming sensitive neuron in the ipsilateral nerve cord whose activity is functionally equivalent to that of the DCMD. This neuron may be the Descending Ipsilateral Movement Detector neuron (DIMD), which responds to the motion of small targets similarly to the DCMD (Rowell, 1971; Burrows and Rowell, 1973). The DIMD has not been identified anatomically, but is known to generate spikes that in some animals are in one-to-one correspondence with those of the DCMD. Furthermore, based on electrophysiological recordings, it is thought to make a monosynaptic connection with the FETi, whose EPSPs summate with those induced by the DCMD. The DIMD is therefore a strong candidate for a mirror symmetric neuron with an equivalent role in generating escape behaviors. Since the response properties of the DIMD to looming stimuli had not yet been characterized, we obtained extracellular recordings simultaneously from both nerve cords in response to the presentation of looming stimuli with various l/|v| values to either eye. We found that the DIMD shows a nearly identical activity profile to the DCMD (Fig. 7C, D). There was no significant difference in the amplitude of the peak firing rate between the two neurons (Supp. Fig. 5A), except at l/|v|= 10 ms. The DCMD peak firing rate however, occurred slightly earlier than the DIMD for small l/|v| values (Supp. Fig. 5B).
The simplest explanation for these results is that the DCMD and the DIMD – given its close resemblance to the DCMD – can interchangeably and equally well mediate jump escape behaviors. According to this hypothesis, because EPSPs elicited in the FETi by these neurons summate, the reduction in jump probability and the increase in variability following nerve cord sectioning would be at least partially explained by the absence of one of them, resulting in delayed co-contraction and a smaller number of subsequent extensor spikes. We conclude that the DCMD is not necessary for jump escape behaviors, provided that the second nerve cord remains intact, since the DIMD can presumably take over its role.
What is the effect of selective contralateral ablation of the DCMD on behavior?
Next, we selectively ablated the DCMD in one nerve cord by filling it intracellularly with 6-carboxy-fluorescein, a phototoxic dye, and shining laser light onto it (Methods). In addition, we sectioned the other nerve cord. This allowed us to determine whether the DCMD is necessary among descending contralateral neurons for the generation of looming-evoked escape behaviors. Since other axons, including the DIMD receiving input from the ipsilateral eye, should remain intact in the spared nerve cord, we used stimulation of the ipsilateral eye as a control (Fig. 8, inset).
Figure 8.
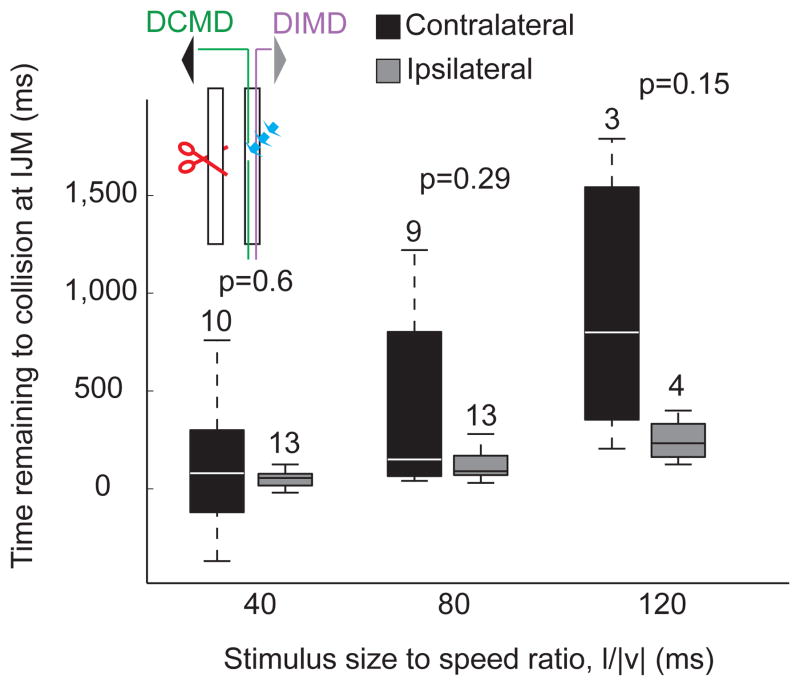
Effect of DCMD laser ablation on the escape behavior (see also Supp. Fig. S6). The timing of the Initial Joint Movement (IJM; box plots), a proxy for the start of flexor motoneuron activity in intact animals, in response to looming stimuli presented to the eye contralateral to the ablated DCMD was not significantly different from control (i.e., when looming stimuli were presented to the eye ipsilateral to the intact nerve cord). The timing of IJM, however, showed more variability (CVipsi=0.8, 0.5, 0.5 and CVcontra=2.68, 1.16, 0.8, for l/|v|=40, 80, and 120 ms, respectively). Inset illustrates the ablation configuration, with the left nerve cord sectioned (red scissors) and the DCMD laser ablated (blue arrows). Black and grey triangles indicate stimulated eyes; DIMD indicates the projection of the DIMD through the intact nerve cord.
We could successfully carry out the ablation procedure in 9 locusts (out of 40 attempts), as evidenced by the selective disappearance of the DCMD spikes from extracellular recordings in response to looming stimuli (Supp. Fig. 6A and B; Methods, Laser Ablation). We could subsequently elicit jumps in 4 of these locusts. An additional 5 animals prepared for, but did not carry out a jump in response to looming stimuli to either eye. Since these experiments were carried out without a telemetry backpack, we analyzed the jump preparation sequence in these nine locusts based on simultaneously acquired video recordings. The timing of the initial joint movement (IJM; see Figs. 1 and 3), which is a proxy for the activation onset of flexor motor neurons in intact animals (Fotowat and Gabbiani, 2007), did not differ when stimulating the eye ipsi- or contralateral to the remaining nerve cord. However, it showed higher variability in response to stimulation of the contralateral eye and a lower correlation with l/|v| (Fig. 8; ρcontra = 0.48, p = 0.009; ρipsi = 0.69, p < 10−9). Three out of the four locusts that jumped did so only in response to stimulation of the eye ipsilateral to the spared cord, but one jumped in response to stimulation of either eye. In this locust, the probability of jumping was slightly lower for contralateral eye stimulation (njump-ipsi = 4, Probjump-ipsi = 33%, njump-contra = 2, Probjump-contra = 20%). The two jumps in response to contralateral eye stimulation occurred 60 and 140 ms after projected collision, considerably later than observed in intact animals for l/|v| = 80 ms (mean: 68 ms before collision; SD=42 ms; nL = 7, nT = 89). Indeed in intact animals, in only two trials for one animal did take-off occur after collision − 2.2% of all trials – with the latest take-off time being 35 ms after collision. In contrast, the two jumps elicited by ipsilateral stimulation at the same l/|v| value occurred 0 and 10 ms before collision, and were thus relatively close to the range observed in intact animals.
Since one locust jumped in response to stimulation of the eye contralateral to the nerve cord where we had ablated the DCMD, this indicates that other contralateral descending neurons respond to looming stimuli (as recently reported by Gray et al., 2010) and are able to activate the motor circuitry generating the jump. In fact, after all nine successful DCMD ablations we could still record multi-unit activity elicited by looming stimuli in the affected nerve cord (Supp. Fig. 6B). The peak of the multi-unit activity, however, occurred significantly later than that of the DCMD (106 ms, difference of medians; pKWT <10−9). In three of the animals that jumped after DCMD laser ablation, including the one that jumped on both sides, we measured the activity of the nerve cord in response to looming stimuli presented to the eye ipsi and contralateral to the remaining nerve cord after the behavioral experiments (Supp. Fig. 6C). The DIMD spikes were detectable as the largest in response to stimulation of the ipsilateral eye, while one or more unidentified units were activated in response to contralateral eye stimulation. We presented looming stimuli with 9 different l/|v| values and compared the timing of the peak multi-unit activity evoked in the contralateral nerve cord to the stimulated eye with that of the DIMD. We found that the peak multi-unit activity occurred later than that of the DIMD (Supp. Fig. 6D). Because the DCMD peak firing rate occurs earlier or around the time of the DIMD peak (Supp. Fig. 5B), we conclude that for all l/|v| values, the peak multi-unit contralateral activity occurs later than the DCMD peak.
These results indicate that, among contralateral descending neurons, the DCMD plays a critical role in the timely triggering of co-contraction and take-off, but probably not in the generation of the initial hind-leg flexion and joint movement. Furthermore, other descending contralateral units can trigger a jump, but given their delayed peak activity, these jumps occur close to, or even after expected collision. Such delayed jumps are rare in intact animals.
Discussion
Using a novel miniature telemetry system, we were for the first time able to record simultaneously the sensory and motor activity contributing to the execution of a complex, multi-stage escape behavior in freely behaving animals. This allowed us to study how variability in the sensory response affects the final motor output on a trial-by-trial basis. Our results suggest that the DCMD neuron contributes to multiple aspects of the behavior through several distinct attributes of its time-varying firing rate. In addition, ablation experiments suggest that, together with the DIMD neuron, the DCMD is an important element of the circuitry mediating timely escape behaviors. We expect that miniature wireless telemetry will contribute to the study of sensorimotor integration during free behavior in other species as well.
Understanding how sensory stimuli are processed by the nervous system to generate complex behaviors in real time is a central goal of systems and computational neuroscience. In this context, the relatively compact nervous system of many invertebrates offers a unique opportunity to study the contribution of single sensory neurons to natural behavior, particularly when they can be reliably identified and the neural circuitry in which they are embedded is well described. Such is the case of the DCMD neuron, whose properties have been characterized for over forty years (Burrows 1996), allowing us to investigate how its visual responses contribute to distinct motor phases of an ongoing behavior.
We found little evidence for an involvement of the DCMD in the initial preparatory movements leading to the jump, while it played an increasingly important role as collision became imminent. Thus, a DCMD firing rate threshold predicted 36% of the variance of co-contraction onset, suggesting that other neurons still play an important role at this stage. Indeed, both proprioceptive feedback and the C interneuron, that receives DCMD input, are expected to contribute to co-contraction onset (Burrows and Pflüger, 1988; Pearson and Roberston, 1981). After the start of co-contraction, we found a very strong correlation between the number of DCMD and extensor spikes (Fig. 4C; Supp. Text), with the FETi firing rate following faithfully that of the DCMD (Supp. Fig. 2B). Thus, co-contraction onset appears to act as a switch that triggers this faithful transmission mode. In contrast, DCMD spikes have previously been thought incapable of generating spikes in the FETi motoneuron (Burrows and Rowell, 1973; Rogers et al., 2007). In those studies, the peak DCMD firing rate was however lower than the threshold we report for triggering co-contraction. The DCMD was more active in our experiments most likely due to: (i) increased arousal in freely behaving animals (Rowell, 1971b); (ii) increased ambient temperature (Methods); (iii) pre-selection of locusts that responded readily to looming stimuli (typically one third of the animals). Additionally, the EPSPs from the DIMD presumably summated with those of the DCMD (Burrows and Rowell, 1973), consistent with our finding that jump probability was reduced by 50% in locusts with one nerve cord sectioned.
The DIMD is thus an important confounding factor when studying the role of the DCMD in the generation of visually guided escape behaviors, as it conveys nearly identical information to motor centers about impending collision. The existence of this neuron and its similarity to the DCMD had been reported early on (Burrows and Rowell, 1973; Rowell, 1971). Yet, its responses to looming stimuli had not been recorded and its function has since been overlooked. In addition, the circuitry generating visually guided escape behaviors is remarkably robust, since elimination of half of the information travelling from the brain to motor centers has little effect on their execution. Thus, assessing the role played by the DCMD using cell-specific laser ablation required simultaneous sectioning of the other nerve cord. These experiments are technically difficult and had a low success rate (4/40 = 10%). In three out of four animals, no jumps were elicited when stimuli were presented contralateral to the laser ablated DCMD. In the remaining one, jumps in response to stimulation of the contralateral eye occurred considerably later than to ipsilateral stimulation. This result is consistent with our finding that the peak activity in remaining contralateral looming sensitive units occurs significantly later as well (Supp. Fig. 6C and D). We conclude that, among contralateral descending neurons, the DCMD is necessary for the accurate timing of the escape behavior. In zebrafish, selective laser ablation of the Mauthner array of neurons, also eliminates “short-latency, high-performance” escape responses, but still leaves fish capable of generating a longer latency and slower escape response, presumably via other neural pathways (Liu and Fetcho, 1999).
We could predict 75% of the trial-to-trial variability of the jump time from the DCMD peak firing time. The time course of the decay in DCMD firing rate following its peak could contribute to it (Fotowat and Gabbiani, 2007). Other potential sources of variability include the DIMD, additional looming sensitive neurons, local interneurons, and sensory feedback (Pearson et al., 1980; Gynther and Pearson 1989; Jellema and Heitler, 1999).
Finally, we found that the number of DCMD spikes from co-contraction onset was highly predictive of jump occurrence. A classifier trained with this attribute performed even better than one trained with the number of extensor spikes. This points to the fact that the DCMD activity controls jump execution not only through activation of the leg extensor motor neurons, but also through other factors, such as the onset of flexor inhibition.
In conclusion, the transformation of sensory activity into the motor program leading to visually guided jumps appears to rely on at least three distinct attributes of a single neuron’s time-varying discharge: a firing rate threshold, the peak firing rate time and the number of spikes from a specific trigger event (co-contraction onset). This ‘multiplexing’ of motor-related information in a sensory neuron’s response could not be evidenced in earlier experiments where behavior and electrophysiology were carried out separately (Fotowat and Gabbiani, 2007), or when animals were restrained to a trackball (Santer et al., 2008). Although our results strongly suggest multiplexing, they do not definitively prove it. This will require specific manipulation of the DCMD activity during ongoing behavior. Multiplexing of sensory information across populations of neurons has been documented earlier, particularly in the vertebrate visual and olfactory system, but its relation to behavior remains to be determined (Meister, 1996; Friedrich et al., 2004; review: Panzeri et al. 2010). In invertebrates, several examples of neurons that contribute to distinct, and sometimes mutually exclusive, motor behaviors have been studied as well. These neurons can be thought of as being ‘multiplexed’, but on a very different time scale as that evidenced here (Kristan and Shaw, 1997). Our finding that distinct aspects of a complex, time-dependent motor behavior can be encoded by distinct attributes of the time-varying firing rate of a single sensory neuron suggests that similar encoding may occur at the sensory-motor interface in other systems, including vertebrates.
Materials and Methods
Wireless Telemetry
We designed and built a custom integrated circuit that performs the amplification, analog to digital conversion, multiplexing, and wireless transmission of four low-noise channels: two for neural and two for muscle recordings (Supp. Fig. 1). The neural (resp. muscle) recordings are amplified with gains of 1,000 (resp. 100), and filtered in the range of 300 Hz – 5.2 kHz (resp. 20 Hz – 280 Hz). A 9-bit analog to digital converter samples them at 11.52 kHz (resp. 1.92 kHz). The digital wireless transmitter operates based on a frequency-shift keying scheme at 920 MHz. The size of the packaged chip is 5×5 mm2 and was mounted on a 13×9 mm2 printed circuit board (PCB). Data from an accelerometer mounted on the PCB was also transmitted (ADXL330, Analog Devices, Norwood, MA; sampling rate: 1.92 kHz, bandwidth: 0–500 Hz). The accelerometer provided high temporal resolution, but saturated for accelerations above ~3.8 gn (gn = 9.8 m/s2). Therefore, we estimated the peak acceleration based on the video recordings. For this purpose, we tracked the position of the locust eye frame-by-frame and computed numerically its second derivative around the time of the peak. Wireless telemetry ran for 2 hours on a pair of 1.5 V batteries (#337, Energizer, St. Louis, MO). The weight of the system including batteries was 0.79 g (1.2 g after connecting and fixing the transmitter to the animal). The receiver captures the transmitted signals via a half-wavelength monopole antenna and relays them to a computer via a USB port through which it is also powered.
Animal Preparation for Wireless Telemetry
We used adult female locusts weighing more than 2.5 g. Locusts were fixed ventral side up on a holder and a rectangular window was cut open on the cuticle of their thorax. Teflon-coated Stablohm wires of 50 μm diameter were used for extracellular recordings (California Fine Wire, Grover Beach, CA). The coating was removed at the desired recording site. A hook-shaped electrode was implanted around one of the nerve cords between the pro- and meso-thoracic ganglia, and the ground and reference electrodes were placed inside the thorax. The cuticle window was then closed and sealed using Vetbond (3M, St. Paul, MN) and beeswax. A pair of electrodes was inserted in the flexor and extensor muscles of the hind leg ipsilateral to the nerve implant, and secured using Vetbond and beeswax. The extensor muscle was impaled dorsally from the outside in segment b, which is innervated by the FETi motorneuron (Hoyle, 1978). The flexor muscle was impaled medially. For each muscle, the reference electrode was inserted 1 mm from the recording electrode. The four muscle electrodes were bundled together inside a polyimide tube (085-1; MicroLumen, Tampa, FL) to minimize their movement and entanglement with the legs. The other end of the implanted electrodes was soldered to miniature connectors (0508 and 3061; Mill-Max, Oyster Bay, NY). The animal was then fixed dorsal side up with electric tape and the wireless transmitter system was attached to the cuticle around the neck using an equal mixture of rosin and beeswax. The connector ends of the electrodes were then soldered to the telemetry system inputs.
Looming Stimuli
Discs approaching on a collision course with the animal were simulated on a computer screen as described previously (Gabbiani et al., 1999; Fotowat and Gabbiani, 2007; monitor refresh rate = 200 fps). Briefly, the instantaneous angular size, θ(t), subtended at one eye by a disk of radius, l, approaching the animal with at constant speed, v, is fully characterized by the ratio, l/|v|, since θ(t)=2 × tan−1 (l/(v×t)). By convention, v<0 for approaching stimuli and t<0 before collision.
Video Recordings
A high-speed digital video camera (IPX-VGA210; Imperx, Boca Raton, FL), equipped with a zoom lens (LIM250M; Kowa, Torrance, CA) was used to record the escape behavior. Recordings were obtained at 100 frames per second with each frame acquisition triggered by alternate frames of the visual stimulation computer.
Behavior with Full Stimulus Expansion
The behavioral setup and conditions were identical to those described earlier (Fotowat and Gabbiani, 2007). Ten locusts equipped with the telemetry system were presented looming stimuli with l/|v|=40, 80, and 120 ms. These values correspond to the lower, middle and upper part of the range eliciting reliable escape behaviors. In 6 locusts, one channel of nerve cord recording was transmitted. In the other 4 locusts, the activity of flexor and extensor muscles was also recorded.
Behavior with Partial Stimulus Expansion
Nine locusts were presented looming stimuli with l/|v| = 40, 80, and 120 ms. The final radius was chosen randomly from 1, 1/2, 1/4, 1/8 and 1/16 of the full size. We identified the smallest final size at which the co-contraction was initiated, and varied it slightly around that value to get a better estimate. Nerve cord, flexor, and extensor muscle activities were recorded and transmitted wirelessly as described above.
Simultaneous Recordings from Both Nerve Cords
The extracellular activity of the nerve cords ipsi- and contralateral to the stimulated eye were recorded simultaneously in 9 fixed locusts at l/|v| = 10–60 (in steps of 10), 80, 100, and 120 ms.
Nerve Cord Ablation Experiments
Looming evoked escape behaviors were studied in 6 locusts, before and after cutting one of their nerve cords. Looming stimuli were presented to the eyes ipsi- and contralateral to the sectioned nerve cord at l/|v| = 40, 80, and 120 ms.
Animal Preparation and Electrophysiology for Laser Ablation
Laser ablation allows to selectively inactivate a single neuron after filling it with a phototoxic dye (Miller and Selverston, 1979; Jacobs and Miller, 1985). Animals were mounted ventral side up on a holder, a hook electrode was implanted around one nerve cord between the pro- and meso-thoracic ganglia, the other nerve cord was sectioned, and the cuticle was sealed back in place. The quality of the extracellular nerve cord recording was then tested; laser ablation was only attempted when it was high (e.g., Supp. Fig. 6A). Next, the locust head was tilted backward and a vertical incision was made in the neck skin, exposing the nerve cords running between the suboesophageal and pro-thoracic ganglia. A small area of the intact nerve cord was de-sheathed using fine forceps. To achieve mechanical stability during intracellular recordings, the nerve cord was raised and secured in place using a pair of polyimide tubes placed under and at the boundary of the de-sheathed area (039-1; MicroLumen, Tampa, FL). Glass electrodes were pulled on a Brown–Flaming micropipette puller (P-97, Sutter Inst., Novato, CA) using thin-wall capillaries with an outer diameter of 1.2 mm (WPI, Sarasota, FL). The tips of the electrodes were filled with 4 μl of 10 mM 6-carboxy-fluorescein (Sigma, St. Louis, MO) and the shafts with 6 μl of a 2 M KAc, 0.5 M KCl solution. The electrode resistances varied between 45 and 50 MΩ. The DCMD axon is located dorso-medially in the nerve cord and was identified based on the 1-1 correspondence with the largest spikes in the extracellular recording. It was filled by electrophoresis for 12 min with currents between -1 and -12 nA. The filling was monitored visually by means of a fluorescence module attached to a stereomicroscope. After filling, the intracellular electrode was removed and the saline level was lowered to minimize the loss of laser power due to light scattering. Laser light was directed onto the axon while the activity of the DCMD was monitored on the extracellular electrode to confirm its eventual laser ablation, typically after 2–5 mins.
Laser Ablation Optical Setup
We used a Cyan Scientific 488 nm, 20 mW, continuous wave laser (Spectra-Physics Laser Division, Newport, Santa Clara, CA). The beam was expanded 10 times using two lenses arranged in a telescope configuration (LB1437-A and LB 1092-A, Thorlabs, Newton, NJ) and directed towards the nerve cord using two mirrors and a focusing lens (10D20DM.5, Newport, LBF254-100-A, Thorlabs).
Behavior and Electrophysiology after Laser Ablation
Because the laser ablation procedure involves a long sequence of technically challenging steps, the overall success rate was low. In fact, to date, in none of the studies that have used laser ablation for selective inactivation of insect neurons has the natural behavior of the animals been tested afterwards (Warzecha et al, 1993; Heitler, 1995; Farrow et al., 2003). In 17 out of 40 attempts, we could successfully ablate the DCMD with minimal apparent damage to the nerve cord. Out of these 17 locusts, 9 reacted to looming stimuli when tested behaviorally, but only 4 jumped in response to them. In these 4 animals, the entire procedure most likely affected only the DCMD, as evidenced by subsequent behavior and electrophysiological recordings (Supp. Fig. 6). Indeed, in 3 of these 4 animals, we recorded robust responses to looming stimuli from the remaining nerve cord several hours (and up to 3 days) after laser ablation. While we cannot exclude non-specific damage in the 5 animals that prepared but did not jump to looming stimuli, their jump preparation was similar to that of the other 4. Thus, pooled results of these 9 animals are presented in Fig. 8. In any case, any non-specific damage in these animals would not affect our conclusions. Our results are consistent with previous reports that laser ablation is selective for the cell that is dye-filled (Miller and Selverston, 1979; Jacobs and Miller, 1985).
Data Analysis
Custom MATLAB software was used for data acquisition and analysis (Mathworks, Natick, MA). The DCMD and motor neuron spikes were detected by thresholding. Estimates of the DCMD and motor neurons’ instantaneous firing rates were computed by convolving individual spike trains with a Gaussian (width: 20 ms) as described earlier (Gabbiani et al., 1999). In some jump trials the nerve recording showed some distortions around the time of the peak firing rate (Supp. Text and Supp. Fig. 7). We estimated that we could have missed up to 3 consecutive DCMD spikes around that time. However, this incident did not significantly change the DCMD peak firing rate amplitude and time. The Kruskal- Wallis test (KWT) was used to compare the medians of populations across different treatments. When a significant difference was found, Tukey’s honestly significant difference criterion was used to perform multiple comparisons between pairs of medians. In all box plots, the whiskers show the extent, + signs depict outliers, and the top and bottom of the box show the upper and lower quartiles of the data. The horizontal bar inside the box shows the median. Least square linear regression was used for all fits. The Pearson’s correlation coefficient is denoted by ρ; associated significance values refer to the null hypothesis ρ = 0. Partial correlations (ρpart) were calculated to estimate the correlation between two of three inter-correlated variables, controlling for the effect of the third. The percentage of variance of a variable explained by a second correlated variable was estimated as the square of their correlation coefficient. Naïve Bayes classification was used to estimate the predictive power of different sensory and motor attributes for the trial outcome (jump vs. no jump). The probability distributions of individual attributes (required for training the classifier) were estimated empirically and non-parametrically. An estimate of the distribution of miss-classification (error; false positive or false negative) rates for each classifier was obtained by training it on half of the data chosen from 100 random data shuffles and testing it on the other half. The performances of the classifiers trained on different attributes were then compared using the KWT with multiple comparisons.
Supplementary Material
Acknowledgments
This work was supported by the AFRL, HFSP, NIMH and NSF. We would like to thank Drs. H. Krapp and J. Maunsell and Mr. P. Jones for comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burrows M. The Neurobiology of an insect brain. Oxford: Oxford University Press; 1996. [Google Scholar]
- Burrows M, Rowell CF. Connections between descending visual interneurons and metathoracic motoneurons in the locust. J Comp Physiol. 1973;85:221–234. [Google Scholar]
- Burrows M, Pflüger H. Positive feedback loops from proprioceptors involved in leg movements of the locust. J Comp Physiol A. 1988;163:425–440. [Google Scholar]
- Camhi JM, Levy A. The code for stimulus direction in a cell assembly in the cockroach. J Comp Physiol A. 1989;165:83–97. doi: 10.1007/BF00613802. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Newsome WT. Estimates of the contribution of single neurons to perception depend on timescale and noise correlation. J Neurosci. 2009;29:6635–48. doi: 10.1523/JNEUROSCI.5179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EP, Maunsell JHR. Dynamics of neuronal responses in macaque mt and vip during motion detection. Nat Neurosci. 2002;5:985–94. doi: 10.1038/nn924. [DOI] [PubMed] [Google Scholar]
- De Lafuente V, Romo R. Neuronal correlates of subjective sensory experience. Nat Neurosci. 2005;8:1698–703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, Krasne FB. Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci. 1999;22:153–61. doi: 10.1016/s0166-2236(98)01340-x. [DOI] [PubMed] [Google Scholar]
- Farrow K, Haag J, Borst A. Input organization of multifunctional motion-sensitive neurons in the blowfly. J Neurosci. 2003;23:9805–9811. doi: 10.1523/JNEUROSCI.23-30-09805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotowat H, Fayyazuddin A, Bellen HJ, Gabbiani F. A novel neuronal pathway for visually guided escape in drosophila melanogaster. J Neurophysiol. 2009;102:875–85. doi: 10.1152/jn.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotowat H, Gabbiani F. Relationship between the phases of sensory and motor activity during a looming-evoked multistage escape behavior. J Neurosci. 2007;27:10047–59. doi: 10.1523/JNEUROSCI.1515-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW, Habermann CJ, Laurent G. Multiplexing using synchrony in the zebrafish olfactory bulb. Nat Neurosci. 2004;7:862–871. doi: 10.1038/nn1292. [DOI] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG, Laurent G. Computation of object approach by a wide-field, motion-sensitive neuron. J Neurosci. 1999;19:1122–41. doi: 10.1523/JNEUROSCI.19-03-01122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Mo C, Laurent G. Invariance of angular threshold computation in a wide-field looming-sensitive neuron. J Neurosci. 2001;21:314–329. doi: 10.1523/JNEUROSCI.21-01-00314.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG, Koch C, Laurent G. Multiplicative computation in a visual neuron sensitive to looming. Nature. 2002;21:320–324. doi: 10.1038/nature01190. [DOI] [PubMed] [Google Scholar]
- Gabbiani F, Cohen I, Laurent G. Time-dependent activation of feed-forward inhibition in a looming-sensitive neuron. J Neurophysiol. 2005;94:2150–2161. doi: 10.1152/jn.00411.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M, Andersen R, Snowden R. Tuning of mst neurons to spiral motions. J Neurosci. 1994;14:54–67. doi: 10.1523/JNEUROSCI.14-01-00054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Blincow E, Robertson RM. A pair of motion-sensitive neurons in the locust encode approaches of a looming object. J Comp Physiol A. 2010 doi: 10.1007/s00359-010-0576-7. [DOI] [PubMed] [Google Scholar]
- Gu Y, Angelaki DE, Deangelis GC. Neural correlates of multisensory cue integration in macaque MSTd. Nat Neurosci. 2008;11:1201–10. doi: 10.1038/nn.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gynther IC, Pearson KG. An evaluation of the role of identified interneurons in triggering kicks and jumps in the locust. J Neurophysiol. 1989;61:45–57. doi: 10.1152/jn.1989.61.1.45. [DOI] [PubMed] [Google Scholar]
- Hatsopoulos N, Gabbiani F, Laurent G. Elementary computation of object approach by wide-field visual neuron. Science. 1995;270:1000–3. doi: 10.1126/science.270.5238.1000. [DOI] [PubMed] [Google Scholar]
- Heitler WJ. Quasi-reversible photo-axotomy used to investigate the role of extensor muscle tension in controlling the kick motor programme of grasshoppers. Eur J Neurosci. 1995;7:981–992. doi: 10.1111/j.1460-9568.1995.tb01086.x. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Distributions of nerve and muscle fibre types in locust jumping muscle. J Exp Biol. 1978;73:205–233. doi: 10.1242/jeb.73.1.205. [DOI] [PubMed] [Google Scholar]
- Ishikane H, Gangi M, Honda S, Tachibana M. Synchronized retinal oscillations encode essential information for escape behavior in frogs. Nat Neurosci. 2005;8:1087–1095. doi: 10.1038/nn1497. [DOI] [PubMed] [Google Scholar]
- Jacobs GA, Miller JP. Functional properties of individual neuronal branches isolated in situ by laser photoinactivation. Science. 1985;228:344–346. doi: 10.1126/science.3983633. [DOI] [PubMed] [Google Scholar]
- Jellema T, Heitler WJ. Central and peripheral control of the trigger mechanism for kicking and jumping in the locust. J Comp Neurol. 1999;404:212–20. [PubMed] [Google Scholar]
- Judge S, Rind FC. The locust dcmd, a movement-detecting neurone tightly tuned to collision trajectories. J Exp Biol. 1997;200:2209–2216. doi: 10.1242/jeb.200.16.2209. [DOI] [PubMed] [Google Scholar]
- Kang H, Nakagawa H. Collision-sensitive neurons in the optic tectum of the bullfrog, Rana catesbeiana. J Neurophysiol. 2010 September 1; doi: 10.1152/jn.01055.2009. [DOI] [PubMed] [Google Scholar]
- Killmann F, Schurmann F. Both electrical and chemical transmission between the ‘lobula giant movement detector’ and the ‘descending contralateral movement detector’ neurons of locusts are supported by electron microscopy. J Neurocytol. 1985;14:637–652. doi: 10.1007/BF01200802. [DOI] [PubMed] [Google Scholar]
- Korn H, Faber DS. The mauthner cell half a century later: a neurobiological model for decision-making? Neuron. 2005;47:13–28. doi: 10.1016/j.neuron.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kristan WB, Shaw BK. Population coding and behavioral choice. Curr Opin Neurobiol. 1997;7:826–831. doi: 10.1016/s0959-4388(97)80142-0. [DOI] [PubMed] [Google Scholar]
- Lewis JE, Kristan WB., Jr A neuronal network for computing population vectors in the leech. Nature. 1998;391:76–79. doi: 10.1038/34172. [DOI] [PubMed] [Google Scholar]
- Lima SQ, Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–52. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999;23:325–335. doi: 10.1016/s0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Marsat G, Pollack GS. A behavioral role for feature detection by sensory bursts. J Neurosci. 2006;26:10542–7. doi: 10.1523/JNEUROSCI.2221-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M. Multineuronal codes in retinal signaling. Proc Nat Acad Sci USA. 1996;93:609–614. doi: 10.1073/pnas.93.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JP, Selverston A. Rapid killing of single neurons by irradiation of intracellularly injected dye. Science. 1979;206:702–704. doi: 10.1126/science.386514. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. ii. differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–20. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- Nienborg H, Cumming BG. Decision-related activity in sensory neurons reflects more than a neuron’s causal effect. Nature. 2009;459:89–92. doi: 10.1038/nature07821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva D, Medan V, Tomsic D. Escape behavior and neuronal responses to looming stimuli in the crab chasmagnathus granulatus (decapoda: Grapsidae) J Exp Biol. 2007;210:865–80. doi: 10.1242/jeb.02707. [DOI] [PubMed] [Google Scholar]
- O’Shea M, Rowell C, Williams J. The anatomy of a locust visual interneurone; the descending contralateral movement detector. J Exp Biol. 1974;60:1–12. [Google Scholar]
- Panzeri S, Brunel N, Logothetis NK, Kayser C. Sensory neural codes using multiplexed temporal scales. Trends Neurosci. 2010;33:111–120. doi: 10.1016/j.tins.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Heitler WJ, Steeves JD. Triggering of locust jump by multimodal inhibitory interneurons. J Neurophysiol. 1980;43:257–78. doi: 10.1152/jn.1980.43.2.257. [DOI] [PubMed] [Google Scholar]
- Pearson K, Robertson R. Interneurons coactivating hindleg flexor and extensor motoneurons in the locust. J Comp Physiol A. 1981;144:391–410. [Google Scholar]
- Peron S, Gabbiani F. Spike frequency adaptation mediates looming stimulus selectivity in a collision-detecting neuron. Nat Neurosci. 2009;12:318–26. doi: 10.1038/nn.XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss T, Osei-Bonsu PE, Weiss SA, Wang C, Faber DS. Neural representation of object approach in a decision-making motor circuit. J Neurosci. 2006;26:3454–64. doi: 10.1523/JNEUROSCI.5259-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rind FC. A chemical synapse between two motion detecting neurones in the locust brain. J Exp Biol. 1984;110:143–67. doi: 10.1242/jeb.110.1.143. [DOI] [PubMed] [Google Scholar]
- Rind FC, Simmons P. Orthopteran dcmd neuron: a reevaluation of responses to moving objects. I. selective responses to approaching objects. J Neurophysiol. 1992;68:1654–1666. doi: 10.1152/jn.1992.68.5.1654. [DOI] [PubMed] [Google Scholar]
- Rogers SM, Krapp HG, Burrows M, Matheson T. Compensatory plasticity at an identified synapse tunes a visuomotor pathway. J Neurosci. 2007;27:4621–33. doi: 10.1523/JNEUROSCI.4615-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci. 2002;22:9475–89. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell C. The orthopteran descending movement detector (dmd) neurones: a characterisation and review. J Comp Physiol A. 1971;73:167–194. [Google Scholar]
- Rowell C. Variable responsiveness of a visual interneurone in the free-moving locust, and its relation to behaviour and arousal. J Exp Biol. 1971b;55:727–747. [Google Scholar]
- Santer R, Rind FC, Stafford R, Simmons P. Role of an identified looming-sensitive neuron in triggering a flying locust’s escape. J Neurophysiol. 2006;95:3391–3400. doi: 10.1152/jn.00024.2006. [DOI] [PubMed] [Google Scholar]
- Santer RD, Yamawaki Y, Rind FC, Simmons PJ. Motor activity and trajectory control during escape jumping in the locust Locusta migratoria. J Comp Physiol A. 2005;191:965–975. doi: 10.1007/s00359-005-0023-3. [DOI] [PubMed] [Google Scholar]
- Santer RD, Yamawaki Y, Rind FC, Simmons PJ. Preparing for escape: an examination of the role of the dcmd neuron in locust escape jumps. J Comp Physiol A. 2008;194:69–77. doi: 10.1007/s00359-007-0289-8. [DOI] [PubMed] [Google Scholar]
- Schlotterer GR. Response of the locust descending movement detector neuron to rapidly approaching and withdrawing visual stimuli. Canad J Zool. 1977;55:1372–1376. [Google Scholar]
- Simmons P. Connexions between a movement-detecting visual interneurone and flight motoneurones of a locust. J Exp Biol. 1980;86:87–97. [Google Scholar]
- Sun H, Frost B. Computation of different optical variables of looming objects in pigeon nucleus rotundus neurons. Nat Neurosci. 1998;1:296–303. doi: 10.1038/1110. [DOI] [PubMed] [Google Scholar]
- Trimarchi JR, Schneiderman AM. Giant fiber activation of an intrinsic muscle in the mesothoracic leg of drosophila melanogaster. J Exp Biol. 1993;177:149–67. doi: 10.1242/jeb.177.1.149. [DOI] [PubMed] [Google Scholar]
- van Hateren JH, Kern R, Schwerdtfeger G, Egelhaaf M. Function and coding in the blowfly H1 neuron during naturalistic optic flow. J Neurosci. 2005;25:4343–52. doi: 10.1523/JNEUROSCI.0616-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Frost BJ. Time to collision is signaled by neurons in the nucleus rotundus of pigeons. Nature. 1992;356:236–8. doi: 10.1038/356236a0. [DOI] [PubMed] [Google Scholar]
- Warzecha AK, Egelhaaf M, Borst A. Neural circuit tuning fly visual interneurons to motion of small objects. I. Dissection of the circuit by pharmacological and photoinactivation techniques. J Neurophysiol. 1993;69:329–339. doi: 10.1152/jn.1993.69.2.329. [DOI] [PubMed] [Google Scholar]
- Wicklein M, Strausfeld NJ. Organization and significance of neurons that detect change of visual depth in the hawk moth manduca sexta. J Comp Neurol. 2000;424:356–76. [PubMed] [Google Scholar]
- Yamamoto K, Nakata M, Nakagawa H. Input and output characteristics of collision avoidance behavior in the frog rana catesbeiana. Brain Behav Evol. 2003;62:201–11. doi: 10.1159/000073272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.