Figure 1.
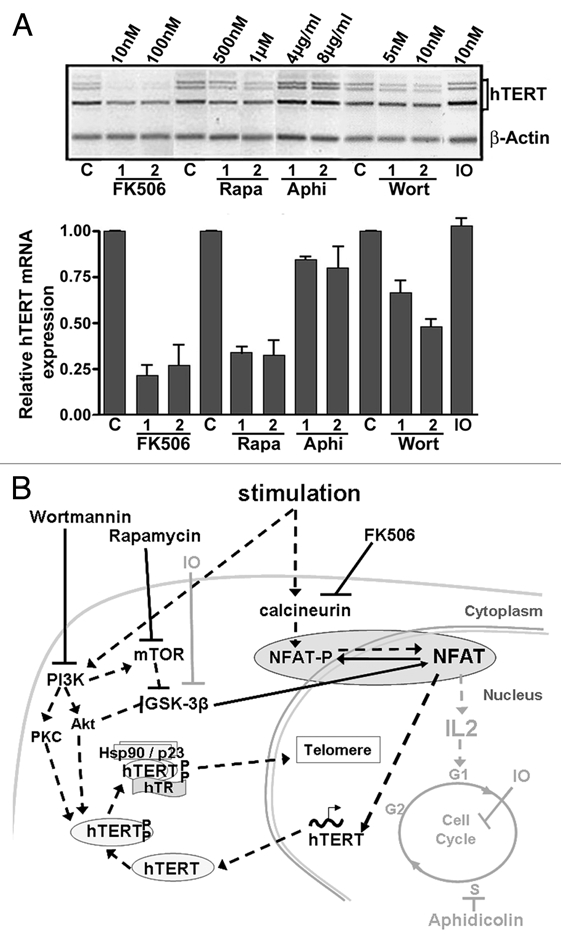
Modulation of hTERT expression during lymphocyte stimulation. (A) hTERT mRNA expression in PBL simultaneously stimulated (1 µg/µl PHA) and treated for 48 h with FK506, Rapamycin (Rapa), aphidicolin (Aphi), wortmannin (Wort) and indirubin-3′-monoxim (IO). hTERT mRNA expression is shown in a representative illustration after semi-quantitative RT-PCR and in a histogram summarizing the results from three independent experiments of real-time quantitative PCR (ratio of hTERT expression to control and normalized to actin expression). The strongest inhibitory effect was observed with FK506, while aphidicolin and IO did not clearly interfere with hTERT mRNA expression. (C: untreated control PBL). (B) Diagram representing lymphocyte stimulation and inhibition pathways. Lymphocyte stimulation activates the phosphatase calcineurin, which rapidly dephosphorylates NFAT, and also activates the PI3K/Akt/mTOR pathway, leading to the inhibition of the GSK-3β activity, a kinase for NFAT. Dephosphorylated NFAT is translocated into the nucleus, where it can exert its transcription factor role for hTERT and cytokines such as IL-2. The kinases Akt and PKC, both activated by PI3K, phosphorylate hTERT, which forms a complex with hTR and the chaperone proteins Hsp90 and p23. This complex is translocated into the nucleus where it can exert its function as a reverse transcriptase in telomere maintenance. FK506 induces the inhibition of calcineurin. Rapamycin and wortmannin inhibit mTOR and PI3K (and thus Akt) respectively, leading to GSK-3β activation. All of these compounds ensure NFAT phosphorylation and its subsequent inactivation. The actions of Aphidicolin and IO that do not interfere with NFAT activity and hTERT mRNA expression are marked in grey. The pathways implicated positively during lymphocyte stimulation are noted by black dashed arrows and those implicated negatively are noted by black solid arrows.
