Abstract
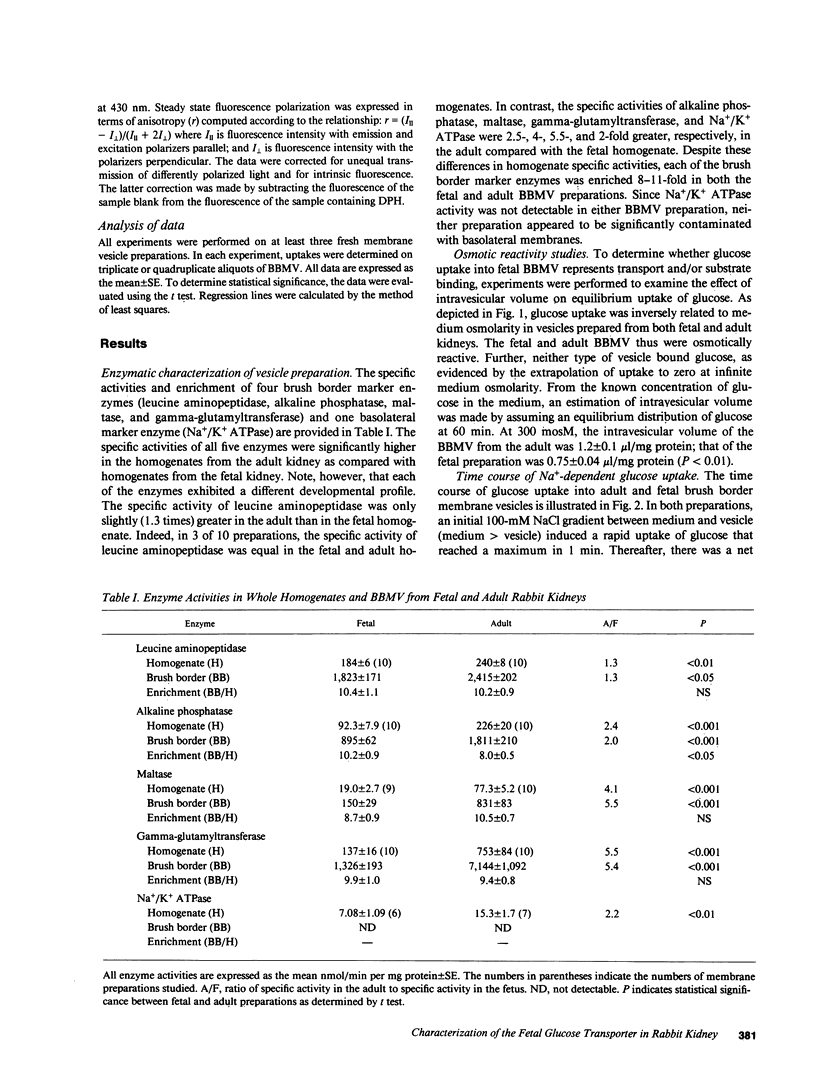
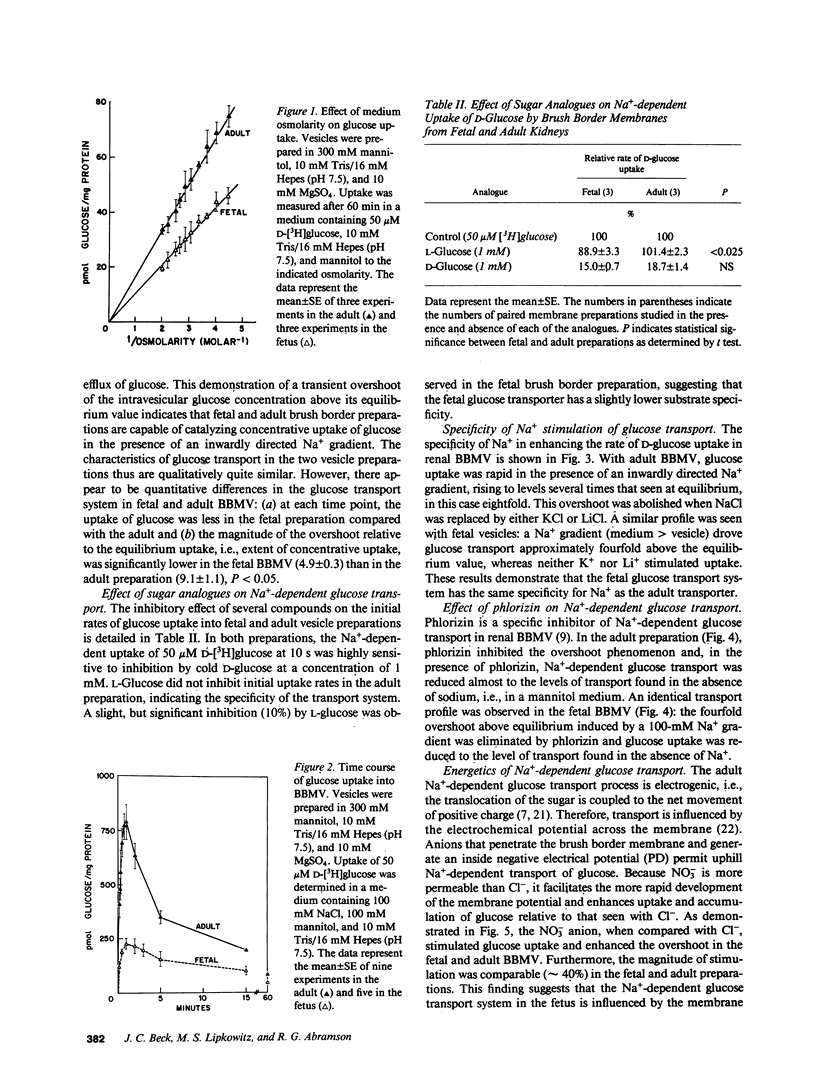
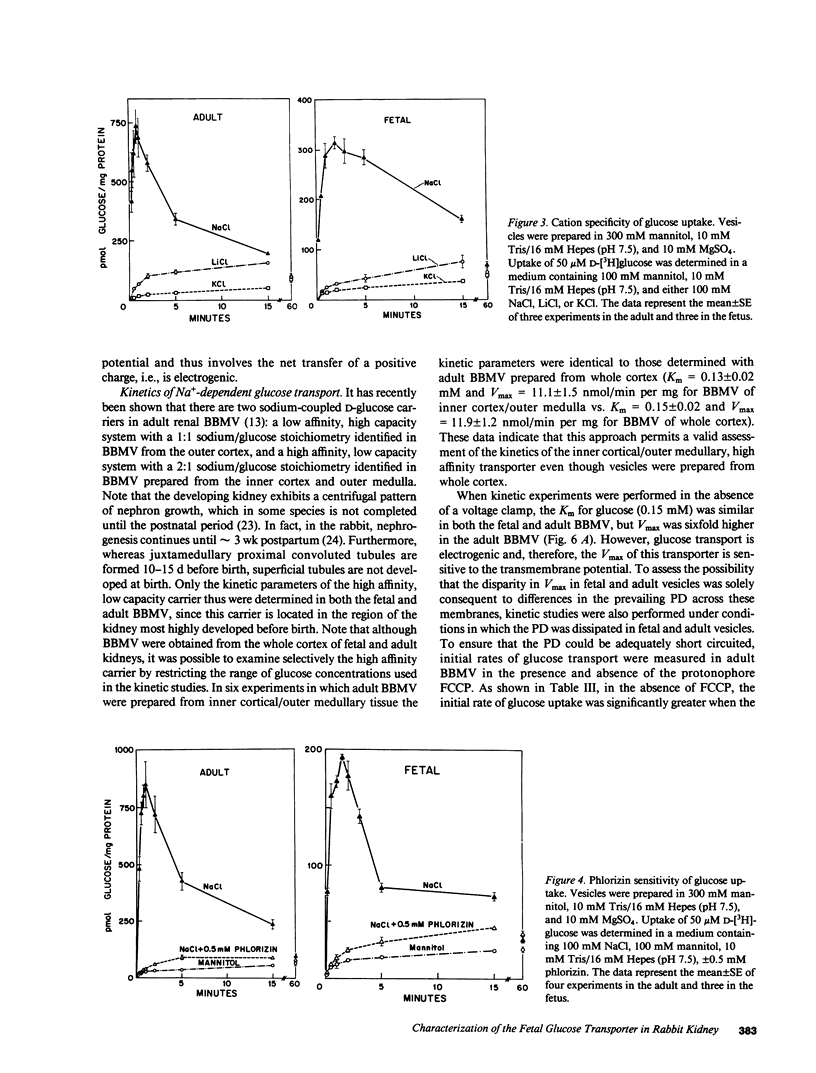
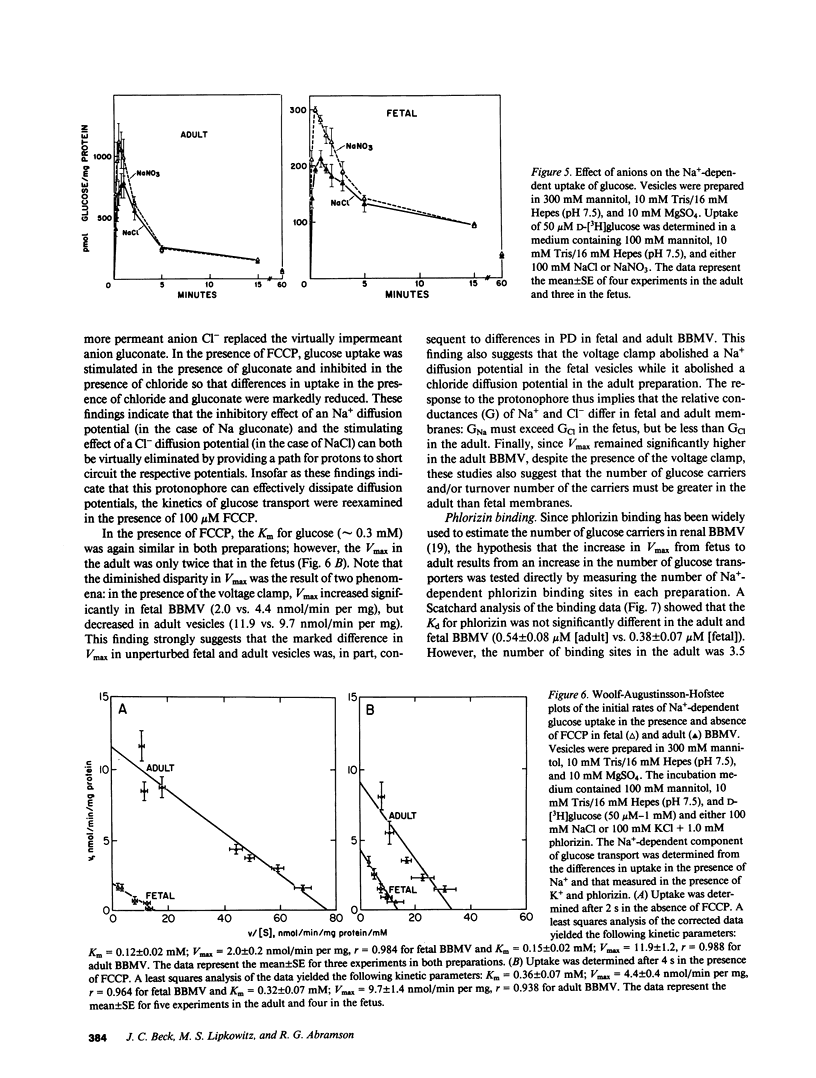
Glucose transport was characterized in rabbit renal brush border membrane vesicles (BBMV) of the fetus late in gestation. Highly purified, osmotically reactive fetal BBMV contained a glucose transporter that was qualitatively indistinguishable from that in the adult: both are concentrative, Na+ dependent, electrogenic, stereospecific, and sensitive to phlorizin. Although the apparent Km for glucose is similar in the fetus and adult, the Vmax is significantly higher in the adult. When the membrane potential was clamped with a protonophore, this difference diminished; however, Vmax remained significantly higher in adult BBMV. This postnatal increase in Vmax was paralleled by a similar increase in the number of phlorizin binding sites. These findings indicate that the maturational increase in glucose transport is, in part, consequent to a more favorable electrical potential for Na+-dependent glucose transport and, in part, the result of the insertion of new transporters. The homogenate activity of several brush border enzymes also demonstrated significant maturational increases. The magnitude of these changes was variable and enzyme dependent. These combined observations suggest that mature expression of membrane proteins (transporters and enzymes) occurs at different stages of development of renal proximal tubule cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER D. P., NIXON D. A. Reabsorption of glucose, fructose and meso-inositol by the foetal and post-natal sheep kidney. J Physiol. 1963 Jul;167:480–486. doi: 10.1113/jphysiol.1963.sp007163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S. Energy-dependence of phlorizin binding to isolated renal microvillus membranes. Evidence concerning the mechanism of coupling between the electrochemical Na+ gradient the sugar transport. J Membr Biol. 1978 Jul 21;42(1):81–98. doi: 10.1007/BF01870395. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Sacktor B. The Na+ gradient-dependent transport of D-glucose in renal brush border membranes. J Biol Chem. 1975 Aug 10;250(15):6032–6039. [PubMed] [Google Scholar]
- Beck J. C., Sacktor B. Energetics of the Na+-dependent transport of D-glucose in renal brush border membrane vesicles. J Biol Chem. 1975 Nov 25;250(22):8674–8680. [PubMed] [Google Scholar]
- Beck J. C., Sacktor B. Membrane potential-sensitive fluorescence changes during Na+-dependent D-glucose transport in renal brush border membrane vesicles. J Biol Chem. 1978 Oct 25;253(20):7158–7162. [PubMed] [Google Scholar]
- Beck J. C., Sacktor B. The sodium electrochemical potential-mediated uphill transport of D-glucose in renal brush border membrane vesicles. J Biol Chem. 1978 Aug 10;253(15):5531–5535. [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney R. W., Gusowski N., Zeilkovic I., Padilla M. Developmental aspects of renal beta-amino acid transport. V: Brush border membrane transport in nursing animals--effect of age and diet. Pediatr Res. 1986 Sep;20(9):890–894. doi: 10.1203/00006450-198609000-00017. [DOI] [PubMed] [Google Scholar]
- Chesney R. W., Gusowski N., Zelikovic I. Developmental aspects of renal beta-amino acid transport. VI. The role of membrane fluidity and phospholipid composition in the renal adaptive response in nursing animals. Pediatr Res. 1987 Aug;22(2):163–167. doi: 10.1203/00006450-198708000-00013. [DOI] [PubMed] [Google Scholar]
- Ghishan F. K., Wilson F. A. Developmental maturation of D-glucose transport by rat jejunal brush-border membrane vesicles. Am J Physiol. 1985 Jan;248(1 Pt 1):G87–G92. doi: 10.1152/ajpgi.1985.248.1.G87. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M. gamma-Glutamyltransferase in kidney brush border membranes. FEBS Lett. 1972 Jan 1;19(4):340–344. doi: 10.1016/0014-5793(72)80075-9. [DOI] [PubMed] [Google Scholar]
- Goldmann D. R., Roth K. S., Langfitt T. W., Jr, Segal S. L-proline transport by newborn rat kidney brush-border membrane vesicles. Biochem J. 1979 Jan 15;178(1):253–256. doi: 10.1042/bj1780253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann D. R., Schlesinger H., Segal S. Isolation and characterization of the brush border fraction from newborn rat renal proximal tubule cells. Biochim Biophys Acta. 1976 Jan 21;419(2):251–260. doi: 10.1016/0005-2736(76)90351-5. [DOI] [PubMed] [Google Scholar]
- Goncharevskaya O. A., Dlouhá H. The development of various generations of nephrons during postnatal ontogenesis in the rat. Anat Rec. 1975 Jul;182(3):367–375. doi: 10.1002/ar.1091820310. [DOI] [PubMed] [Google Scholar]
- Heidrich H. G., Kinne R., Kinne-Saffran E., Hannig K. The polarity of the proximal tubule cell in rat kidney. Different surface charges for the brush-border microvilli and plasma membranes from the basal infoldings. J Cell Biol. 1972 Aug;54(2):232–245. doi: 10.1083/jcb.54.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E., Weinstein A. M. The overshoot phenomenon in cotransport. Biochim Biophys Acta. 1984 Sep 19;776(1):83–91. doi: 10.1016/0005-2736(84)90253-0. [DOI] [PubMed] [Google Scholar]
- Hise M. K., Weinman E. J. Physical properties of the rat renal brush border membrane during growth. Pflugers Arch. 1986 Feb;406(2):234–236. doi: 10.1007/BF00586689. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LeLièvre-Pégorier M., Geloso J. P. Otogeny of sugar transport in fetal rat kidney. Biol Neonate. 1980;38(1-2):16–24. doi: 10.1159/000241321. [DOI] [PubMed] [Google Scholar]
- Lelievre-Pegorier M., Jean T., Ripoche P., Poujeol P. Transport of phosphate, D-glucose, and L-valine in newborn rat kidney brush border. Am J Physiol. 1983 Sep;245(3):F367–F373. doi: 10.1152/ajprenal.1983.245.3.F367. [DOI] [PubMed] [Google Scholar]
- Linshaw M. A., Welling L. W., Bauman C. A. Basolateral membrane properties of juxtamedullary proximal tubule in newborn rabbit. Am J Physiol. 1986 Aug;251(2 Pt 2):F208–F213. doi: 10.1152/ajprenal.1986.251.2.F208. [DOI] [PubMed] [Google Scholar]
- Lipkowitz M. S., Abramson R. G. Ionic permeabilities of rat renal cortical brush-border membrane vesicles. Am J Physiol. 1987 Apr;252(4 Pt 2):F700–F711. doi: 10.1152/ajprenal.1987.252.4.F700. [DOI] [PubMed] [Google Scholar]
- Medow M. S., Roth K. S., Goldmann D. R., Ginkinger K., Hsu B. Y., Segal S. Developmental aspects of proline transport in rat renal brush border membranes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7561–7564. doi: 10.1073/pnas.83.19.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medow M. S., Segal S. Age related changes in fluidity of rat renal brushborder membrane vesicles. Biochem Biophys Res Commun. 1987 Feb 13;142(3):849–856. doi: 10.1016/0006-291x(87)91491-4. [DOI] [PubMed] [Google Scholar]
- Merlet-Benichou C., Pegorier M., Muffat-Joly M., Augeron C. Functional and morphologic patterns of renal maturation in the developing guinea pig. Am J Physiol. 1981 Dec;241(6):F618–F624. doi: 10.1152/ajprenal.1981.241.6.F618. [DOI] [PubMed] [Google Scholar]
- PERRY J. S., STANIER M. W. The rate of flow of urine of foetal pigs. J Physiol. 1962 May;161:344–350. doi: 10.1113/jphysiol.1962.sp006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang K. Y., Bresson J. L., Walker W. A. Development of the gastrointestinal mucosal barrier. Evidence for structural differences in microvillus membranes from newborn and adult rabbits. Biochim Biophys Acta. 1983 Jan 5;727(1):201–208. doi: 10.1016/0005-2736(83)90385-1. [DOI] [PubMed] [Google Scholar]
- Robillard J. E., Sessions C., Kennedy R. L., Smith F. G., Jr Maturation of the glucose transport process by the fetal kidney. Pediatr Res. 1978 May;12(5):680–684. doi: 10.1203/00006450-197805000-00013. [DOI] [PubMed] [Google Scholar]
- Schwarz S. M., Hostetler B., Ling S., Mone M., Watkins J. B. Intestinal membrane lipid composition and fluidity during development in the rat. Am J Physiol. 1985 Feb;248(2 Pt 1):G200–G207. doi: 10.1152/ajpgi.1985.248.2.G200. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Heterogeneity of sodium-dependent D-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am J Physiol. 1982 Apr;242(4):F406–F414. doi: 10.1152/ajprenal.1982.242.4.F406. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Silverman M. Interaction of phlorizin and sodium with the renal brush-border membrane D-glucose transporter: stoichiometry and order of binding. J Membr Biol. 1981 Jan 30;58(1):43–55. doi: 10.1007/BF01871033. [DOI] [PubMed] [Google Scholar]


