Figure 1.
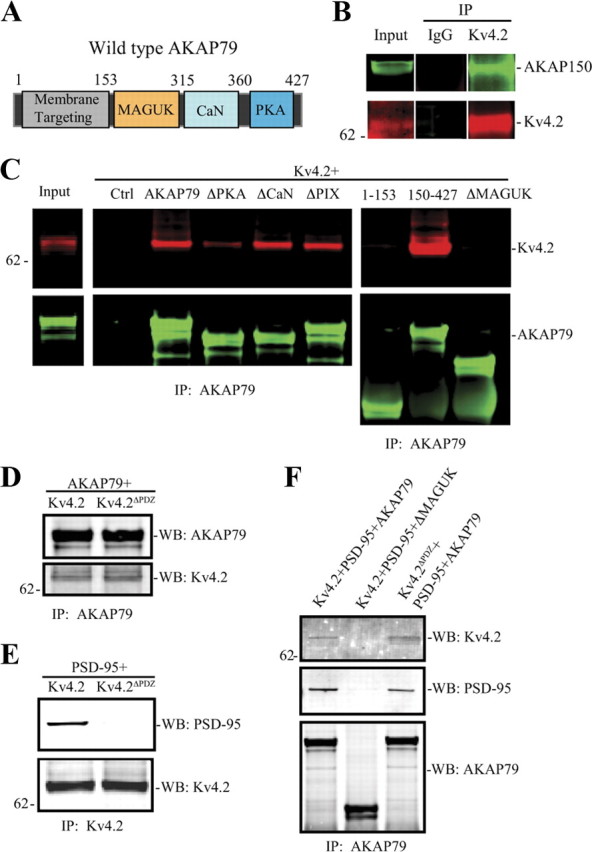
The MAGUK-binding domain of AKAP79/150 is necessary for its interaction with Kv4.2. We used AKAP79 or Kv4.2 deletion mutations to map their interaction. A, Schematic diagram of wild-type human AKAP79 showing the relative positions of various binding domains. B, Native co-IP from rat brain shows endogenous Kv4.2 interacts with AKAP150. C, Co-IP assay in COS7 cells cotransfected Kv4.2 with control, or AKAP79 or AKAP79 deletion mutations. Results show that the MAGUK-binding region (153–315) on AKAP79 is necessary for its interaction with Kv4.2. D, Co-IP of AKAP79 with Kv4.2 or its PDZ domain deletion (Kv4.2ΔPDZ) shows that the binding between the two proteins appears to be direct rather than through a MAGUK intermediate because the PDZ domain of Kv4.2 is not required for interaction. E, PSD-95 with Kv4.2 or its PDZ domain deletion (Kv4.2ΔPDZ) showing that the PDZ domain of Kv4.2 is required for interaction with PSD-95. F, PSD-95 cotransfected with Kv4.2 and AKAP79, Kv4.2, and AKAP79 MAGUK deletion mutation, or Kv4.2ΔPDZ and AKAP79. Cell lysates were pulled down by anti-AKAP79, anti-Kv4.2 antibody, or nonspecific IgG, and probed with anti-Kv4.2 antibody (1:2000), AKAP150 (1:1000, Millipore; for native co-IP), anti-AKAP79 antibody (1:2000), or PSD-95 (1:1000), and visualized by anti-mouse Alexa Fluor 680 and anti-rabbit Alexa Fluor 800 secondary antibody, respectively.
