Figure 6.
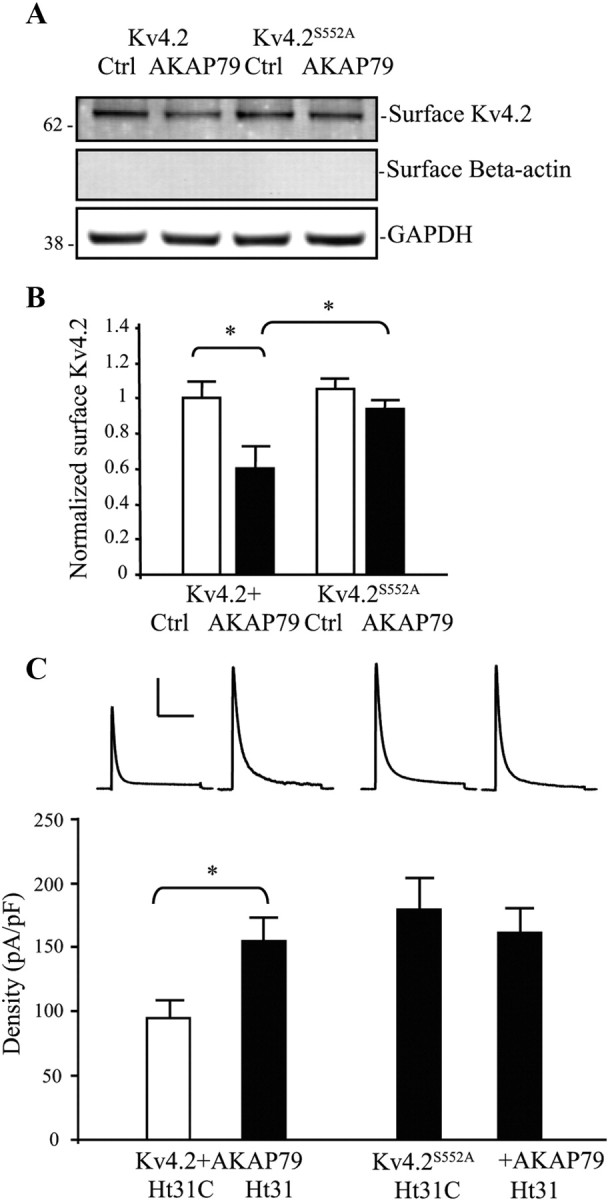
AKAP79 regulation of Kv4.2 trafficking requires Kv4.2 PKA phosphorylation site S552. A, COS7 cells cotransfected Kv4.2 or Kv4.2S552A and either control or AKAP79. Surface proteins were labeled with NHS-SS-Biotin and probed with mouse anti-Kv4.2 (1:2000). Cells coexpressing Kv4.2 and AKAP79 showed decreased surface expression of Kv4.2 compared with control. Surface expression of the Kv4.2 phospho-mutant S552A (Kv4.2S552A) was not changed by AKAP79 coexpression. GAPDH served as a loading control. β-Actin is labeled as negative surface control. B, Pooled data normalized to total Kv4.2 expression show that AKAP79 coexpression decreased surface Kv4.2 expression by ∼40% (n = 5, p < 0.05), and without a significant effect on Kv4.2S552A (n = 5, *p < 0.05). Error bars represent SEM. C, In electrophysiological recordings from COS7 cells, peak current density of Kv4.2-mediated currents increased (∼1.5-fold) by coexpression of AKAP79 with the phospho-mutant Kv4.2S552A (n = 10) compared with Kv4.2 (n = 10). Ht31 application (15 min) significantly increased Kv4.2-mediated peak current density in the neurons cotransfected with Kv4.2 and AKAP79 (n = 10, p < 0.05), but this peak current density enhancement by Ht31 was not found in cells expressing Kv4.2S552A and AKAP79 (n = 13, p < 0.05). Error bars represent SEM. Calibration: 200 pA, 200 ms.
