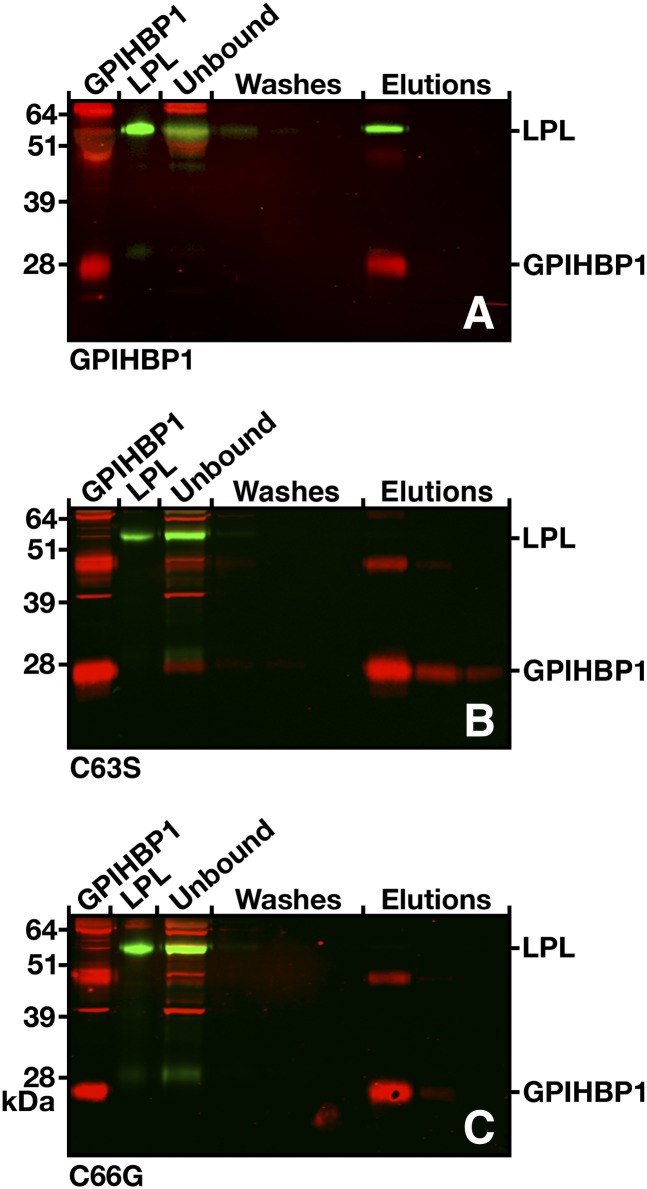
Fig. 9.
Elution of human LPL together with soluble wild-type GPIHBP1 or the soluble forms of the mutants C65S and C68G from agarose beads coated with anti-GPIHBP1 monoclonal antibody (11A12). V5-tagged human LPL was mixed with the different forms of soluble mouse GPIHBP1, along with antibody 11A12-coated beads. After three washes, the bound proteins were eluted with 0.1 M glycine, pH 2.5. GPIHBP1 and LPL in the starting material, the unbound fraction, the washes, and elution fractions were detected by Western blotting. In all cases, most of the GPIHBP1 protein was captured by the antibody 11A12-coated beads, but LPL was coeluted only in the presence of wild-type GPIHBP1 (A). With the GPIHBP1 mutants (B and C), no LPL was detectable in the eluates.