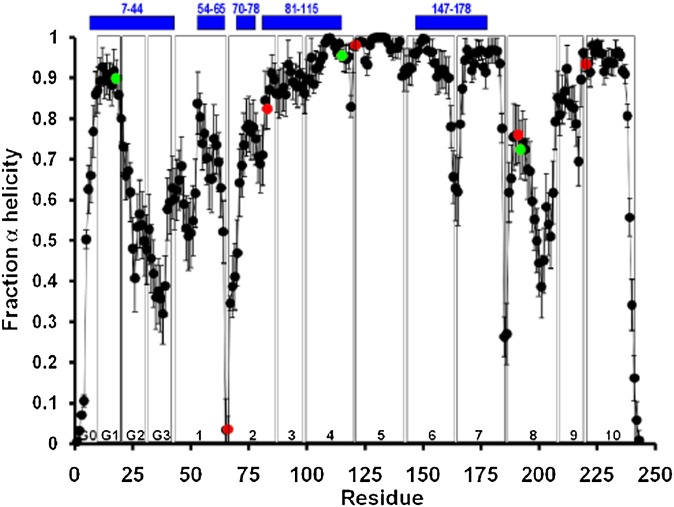
Fig. 4.
Locations of the eight completely conserved residues relative to the predicted α helicity for dimeric lipid-associated and monomeric lipid-free apoA-I are shown (56). Average local (per residue) changes in lipid-associated fractional α helicity (means ± 1 SEM) during the last 10 ns of the MDSA protocol are plotted for 16 160:24:2 particle replicas. Green data points represent positions of the three completely conserved Tyr residues, Y18, Y115, and Y192. Red data points represent positions of the other five completely conserved residues, P66, R83, P121, E191, and P220. The positions of five helical domains predicted for monomeric lipid-free apoA-I by hydrogen exchange-mass spectrometry (56) are represented as blue bars at the top of the figure. Vertical boxes in the figure denote the positions of each of the 10 helical and N-terminal G* repeats.