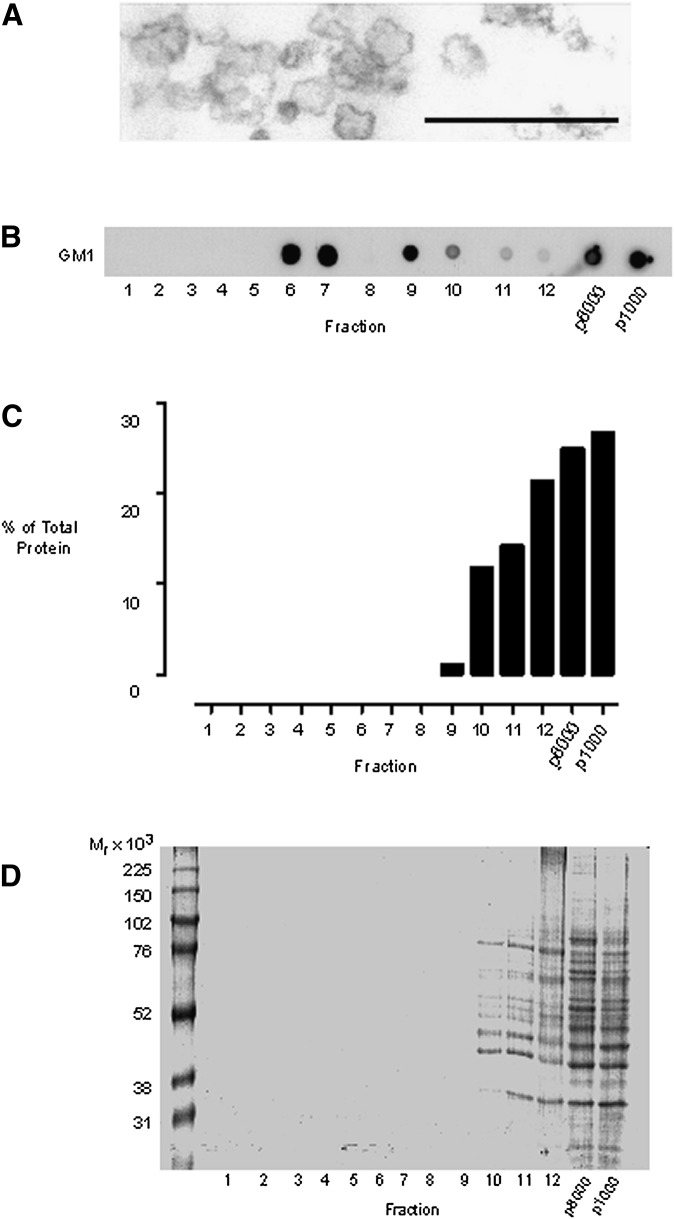
Fig. 2.
Detergent-free isolation of a buoyant-membrane fraction enriched for GM1 lipid. Cells were disrupted by gentle Dounce homogenization, and a nuclear pellet obtained by centrifuging the homogenate at 1,000 g. The 1,000 g pellet was resuspended in 1 ml of homogenization buffer to give the p1000 fraction. The postnuclear supernatant was centrifuged at 8,000 g for 20 min, and the pellet was resuspended in 1 ml of homogenization buffer to form the p8000 fraction. The 8,000 g supernatant was subjected to carbonate addition, sonicated, and bottom loaded on a 40-35-5% (w/v) discontinuous sucrose gradient. The gradient was subjected to overnight ultracentrifugation, and 12 × 1 ml gradient fractions were collected from the top of the tube. (A) Electron micrograph demonstrating that the probe sonication conditions employed resulted in the generation of vesicles with diameters in the region of 50-200 nm. Scale bar 500 nm. (B) Dot blots showing the gradient distribution of GM1 glycosphingolipid, detected with HRP-conjugated cholera toxin B subunit. (C) Analyses of the protein content of the subcellular fractions using the Bradford assay. (D) Colloidal Coomassie blue staining of SDS-PAGE separated proteins from equal-volume samples from each subcellular fraction.