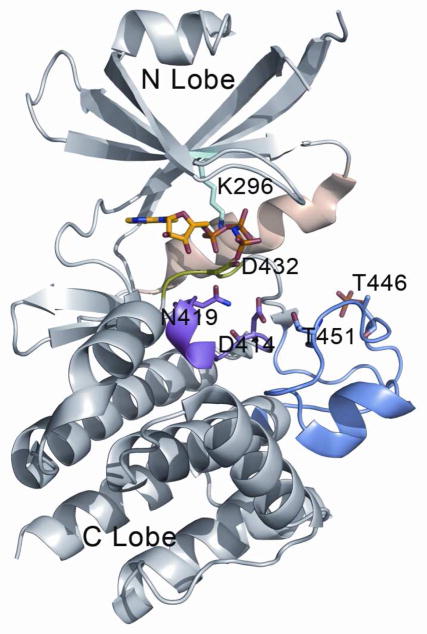
Figure 3.
Binding mode of AMP-PNP with PKR. Kinase domain of PKR are showed in gray, magnesium binding loop in green, catalytic loop in magenta, activation loop in blue and αC helix in wheat. Important residues in the nucleotide binding cavity are shown in sticks and bound the ATP analog adenylyl imidodiphosphate (AMP-PNP) are colored in yellow for the carbon atoms. The figure was generated using the following structures: PKR:AMP-PNP:eIF2a (PDB id = 2A19).