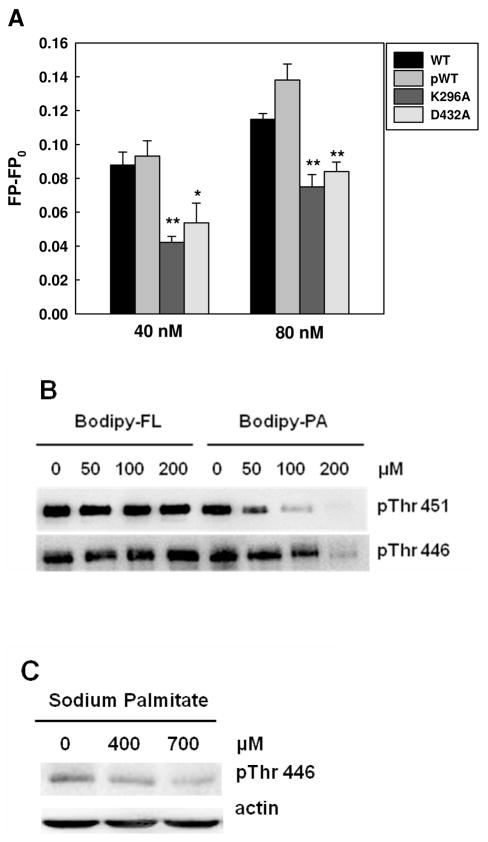
Figure 6.
Inhibitory effects of PA on PKR phosphorylation. (A) Fluorescence polarization measurement of PKR mutants. 10 nM of Bodipy-PA was added to 40 and 80 nM of wild-type PKR proteins (phosphorylated and unphosphorylated) and PKR mutants in PBS buffer. After 5 min of incubation, the fluorescence polarization was measured at an excitation wavelength of 488 nm and an emission wavelength of 520 nm using a spectrofluorometer. * (p<0.05) and ** (p<0.001) indicate statistically different to wild-type proteins. (B) Competition assay with Western blot analysis. 2 μM of PKR-WT was incubated with Bodipy-C3 or Bodipy-C16 at the indicated concentrations in phosphorylation buffer (10 mM HEPES (pH 7.5), 50 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, and 1mM DTT) for 20 min. Autophosphorylation of WT PKR was performed by adding 100 μM of ATP at room temperature for 30 min. To stop the autophosphorylation reaction, 5X SDS-PAGE sample buffer was added to the reactions, followed by heating at 95°C for 5 min. The reactions were loaded onto 10 % Tris/glycine SDS-polyacrylamide gel and detected for PKR phosphorylation at Thr451 and Thr446 by Western blot analysis. (C) Effect of palmitate on the phosphorylated Thr446 (see (22) for Thr451 results). HepG2 cells were cultured in regular medium until reaching 90% confluency and then exposed to 400 and 700 μM for 24 hr. 2% BSA was used as a negative control. After treatment, the cells were harvested and western blot analysis was performed to detect the level of phosphorylated PKR.