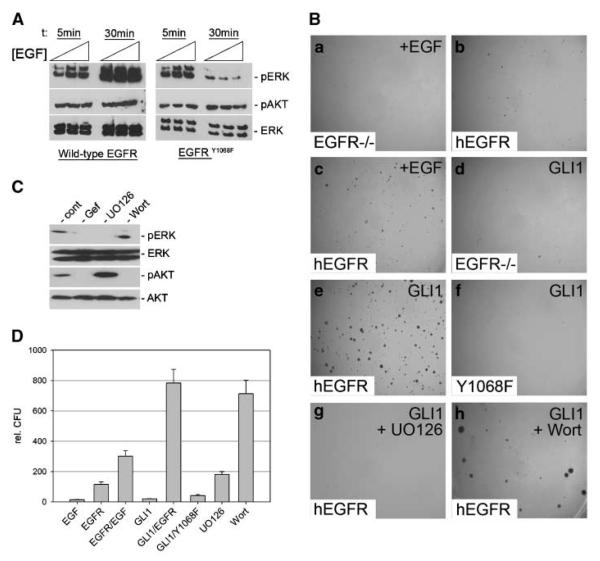
Figure 2.
Requirement of MEK/ERK function for transformation by synergistic EGFR and GLI1 activity. A, Western blot analysis of Egfr−/− cells reconstituted with wild-type human EGFR or EGFRY1068F and stimulated with different concentrations of EGF (0.5, 1, and 5 ng/mL) for 5 and 30 min, respectively. B, EGFR-deficient mouse embryonic fibroblasts treated with 10 ng/mL EGF (a). b–h, EGFR-deficient mouse embryonic fibroblasts either reconstituted with human wild-type EGFR (b, c, e, g, h) or with the GRB2-binding mutant EGFRY1068F (f) or expressing GLI1 only (d). Reconstitution with functional EGFR was validated by high-dosage EGF treatment (10 ng/mL) known to induce anchorage-independent growth (c). d, no transformation by GLI1 in the absence of EGFR. e, transformation by wild-type EGFR in combination with GLI1 activation. Transformation is lost upon reconstitution of EGFR−/− cells with EGFRY1068F (f)orby pharmacologic inhibition of MEK function (UO126 treatment; g) but not by inhibition of PI3K/AKT by wortmannin treatment (h). C, Western blot analysis of Egfr−/− mouse embryonic fibroblasts reconstituted with wild-type human EGFR and treated with EGF showing efficient inhibition of MEK/ERK (pERK) and PI3K/AKT (pAKT) activation by UO126 and wortmannin treatment, respectively. D, quantitative analysis of assays shown in B. Wort, wortmannin; Gef, gefitinib.