Fig. 4.
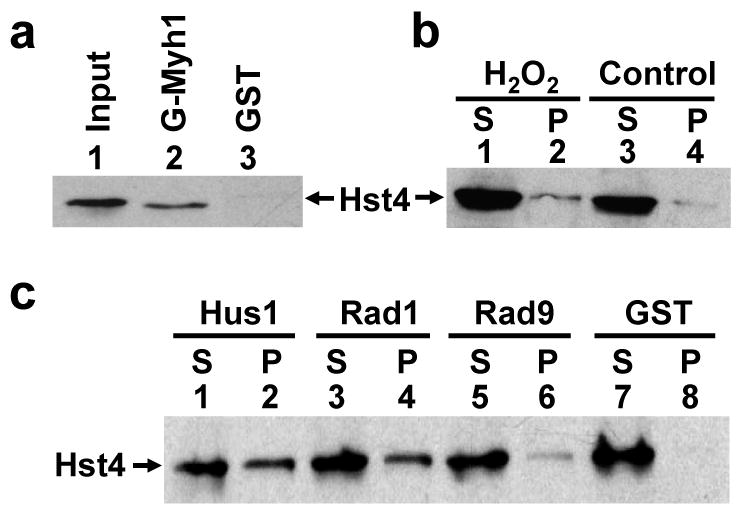
SpHst4 interacts with SpMyh1 and the Rad9/Rad1/Hus1 subunits. (a) Pull-down of SpHst4 by GST-SpMyh1. GST-SpMyh1 (lane 2), and GST alone (lane 3) were immobilized to glutathione-Sepharose and incubated with extracts from Hu1500 containing SpHst4-Myc. The pellets were fractionated by a 10% SDS-PAGE followed by Western blot analysis with the c-Myc antibody. Lane 1 contains 10% of the input yeast extracts. (b) The interaction of SpHst4 and SpMyh1 was enhanced following oxidative stress. Hu1500 cells were exposed to 5 mM H2O2 for 30 min and recovered for 1 hour or left untreated (control). Total soluble cell extracts were prepared and analyzed by immunoprecipitation with antibody against SpMyh1. Western blotting was performed to detect SpHst4. S represents the supernatant and P represents the pellet. (c) Pull-down of SpHst4 by GST-SpHus1, GST-SpRad1, and GST-SpRad9. GST-SpHus1 (lanes 1 and 2), GST-SpRad1 (lanes 3 and 4), GST-SpRad9 (lanes 5 and 6), and GST alone (lanes 7 and 8) were immobilized to glutathione-sepharose and incubated with purified SpHst4-Myc. The supernatants (S, 10%) and pellets were fractionated by a 10% SDS-PAGE followed by Western blot analysis with the c-Myc antibody.
