Abstract
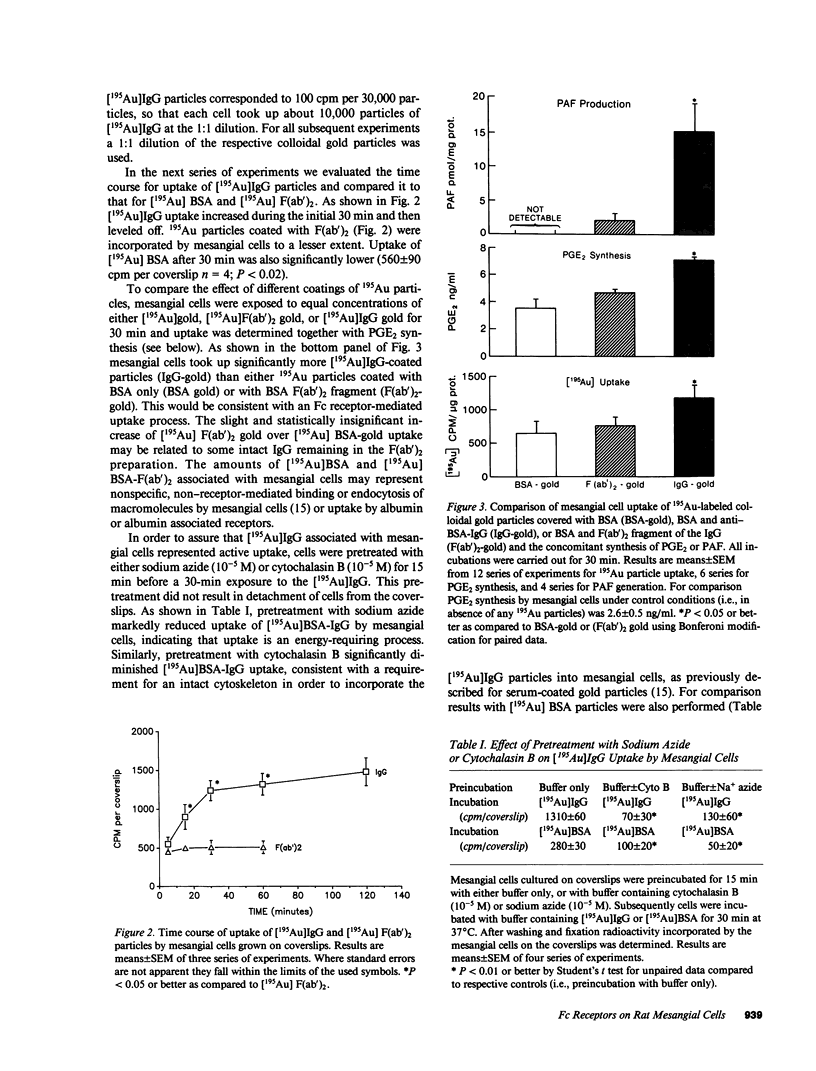
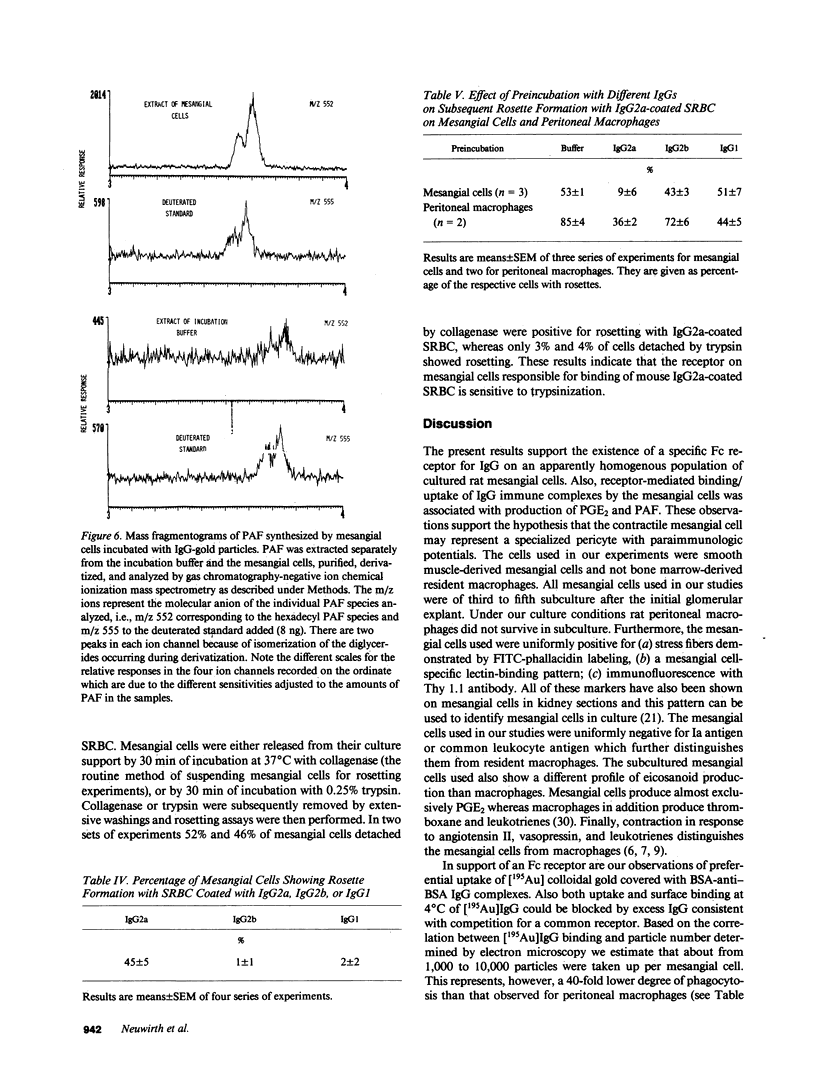
The possibility of Fc-dependent uptake of IgG immune complexes was examined in subcultured rat mesangial cells free of monocytes. 195Au-labeled colloidal gold particles were coated either with BSA only or with BSA followed by rabbit anti-BSA-IgG or the F(ab')2 fragment of the IgG. Mesangial cells preferentially took up 195Au particles covered with BSA-anti-BSA-IgG over those covered with BSA or the F(ab')2 fragment. This uptake was a time-dependent and saturable process inhibitable by sodium azide or cytochalasin B. Using phase-contrast microscopy in the light reflectance mode, it was established that essentially all mesangial cells took up IgG-coated gold particles. By electron microscopy the process was shown to consist of vesicular uptake with delivery to endosomes. Mesangial binding-uptake of the IgG-covered particles was associated with stimulation of PGE2 synthesis and production of platelet-activating factor, a lipid mediator of inflammation. To characterize the potential Fc receptor for IgG we used the rosetting technique with sheep red blood cells coated with IgG subclass-specific mouse monoclonal antibodies. 50% of mesangial cells exhibited rosetting with red cells coated with mouse IgG2a, whereas negligible rosetting was observed with IgG2b or IgG1. Competition experiments confirmed the specificity of IgG2a binding. We conclude that cultured rat mesangial cells exhibit specific receptors for IgG and that occupancy of Fc receptors results in endocytosis and is associated with generation of PGE2 and platelet-activating factor. These observations may be of significance for immune-mediated glomerular diseases.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnoux B., Duval D., Benveniste J. Release of platelet-activating factor (PAF-acether) from alveolar macrophages by the calcium ionophore A23187 and phagocytosis. Eur J Clin Invest. 1980 Dec;10(6):437–441. doi: 10.1111/j.1365-2362.1980.tb02082.x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baud L., Hagege J., Sraer J., Rondeau E., Perez J., Ardaillou R. Reactive oxygen production by cultured rat glomerular mesangial cells during phagocytosis is associated with stimulation of lipoxygenase activity. J Exp Med. 1983 Dec 1;158(6):1836–1852. doi: 10.1084/jem.158.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud L., Perez J., Ardaillou R. Dexamethasone and hydrogen peroxide production by mesangial cells during phagocytosis. Am J Physiol. 1986 Apr;250(4 Pt 2):F596–F604. doi: 10.1152/ajprenal.1986.250.4.F596. [DOI] [PubMed] [Google Scholar]
- Baud L., Sraer J., Delarue F., Bens M., Balavoine F., Schlondorff D., Ardaillou R., Sraer J. D. Lipoxygenase products mediate the attachment of rat macrophages to glomeruli in vitro. Kidney Int. 1985 Jun;27(6):855–863. doi: 10.1038/ki.1985.92. [DOI] [PubMed] [Google Scholar]
- Camazine S. M., Ryan G. B., Unanue E. R., Karnovsky M. J. Isolation of phagocytic cells from the rat renal glomerulus. Lab Invest. 1976 Oct;35(4):315–326. [PubMed] [Google Scholar]
- Camussi G. Potential role of platelet-activating factor in renal pathophysiology. Kidney Int. 1986 Feb;29(2):469–477. doi: 10.1038/ki.1986.23. [DOI] [PubMed] [Google Scholar]
- De Waele M., De Mey J., Moeremans M., De Brabander M., Van Camp B. Immunogold staining method for the light microscopic detection of leukocyte cell surface antigens with monoclonal antibodies: its application to the enumeration of lymphocyte subpopulations. J Histochem Cytochem. 1983 Mar;31(3):376–381. doi: 10.1177/31.3.6186731. [DOI] [PubMed] [Google Scholar]
- Diamond B., Bloom B. R., Scharff M. D. The Fc receptors of primary and cultured phagocytic cells studied with homogeneous antibodies. J Immunol. 1978 Oct;121(4):1329–1333. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Rosset J., Bauer H. Colloidal gold granules as markers for cell surface receptors in the scanning electron microscope. Experientia. 1975 Oct 15;31(10):1147–1149. doi: 10.1007/BF02326761. [DOI] [PubMed] [Google Scholar]
- LATTA H., MAUNSBACH A. B., MADDEN S. C. The centrolobular region of the renal glomerulus studied by electron microscopy. J Ultrastruct Res. 1960 Dec;4:455–472. doi: 10.1016/s0022-5320(60)80033-0. [DOI] [PubMed] [Google Scholar]
- Latta H., Fligiel S. Mesangial fenestrations, sieving, filtration, and flow. Lab Invest. 1985 Jun;52(6):591–598. [PubMed] [Google Scholar]
- Lee S., Vernier R. L. Immunoelectron microscopy of the glomerular mesangial uptake and transport of aggregated human albumin in the mouse. Lab Invest. 1980 Jan;42(1):44–58. [PubMed] [Google Scholar]
- Leiper J. M., Thomson D., MacDonald M. K. Uptake and transport of Imposil by the glomerular mesangium in the mouse. Lab Invest. 1977 Nov;37(5):526–533. [PubMed] [Google Scholar]
- Lovett D. H., Sterzel R. B. Cell culture approaches to the analysis of glomerular inflammation. Kidney Int. 1986 Aug;30(2):246–254. doi: 10.1038/ki.1986.176. [DOI] [PubMed] [Google Scholar]
- Mancilla-Jimenez R., Bellon B., Kuhn J., Belair M. F., Rouchon M., Druet P., Bariety J. Phagocytosis of heat-aggregated immunoglobulins by mesangial cells: an immunoperoxidase and acid phosphatase study. Lab Invest. 1982 Mar;46(3):243–253. [PubMed] [Google Scholar]
- Pradelles P., Grassi J., Maclouf J. Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: an alternative to radioimmunoassay. Anal Chem. 1985 Jun;57(7):1170–1173. doi: 10.1021/ac00284a003. [DOI] [PubMed] [Google Scholar]
- Ramesha C. S., Pickett W. C. Platelet-activating factor and leukotriene biosynthesis is inhibited in polymorphonuclear leukocytes depleted of arachidonic acid. J Biol Chem. 1986 Jun 15;261(17):7592–7595. [PubMed] [Google Scholar]
- Schlondorff D., Aynedjian H. S., Satriano J. A., Bank N. In vivo demonstration of glomerular PGE2 responses to physiological manipulations and experimental agents. Am J Physiol. 1987 Apr;252(4 Pt 2):F717–F723. doi: 10.1152/ajprenal.1987.252.4.F717. [DOI] [PubMed] [Google Scholar]
- Schlondorff D., DeCandido S., Satriano J. A. Angiotensin II stimulates phospholipases C and A2 in cultured rat mesangial cells. Am J Physiol. 1987 Jul;253(1 Pt 1):C113–C120. doi: 10.1152/ajpcell.1987.253.1.C113. [DOI] [PubMed] [Google Scholar]
- Schlondorff D., Satriano J. A., Hagege J., Perez J., Baud L. Effect of platelet-activating factor and serum-treated zymosan on prostaglandin E2 synthesis, arachidonic acid release, and contraction of cultured rat mesangial cells. J Clin Invest. 1984 Apr;73(4):1227–1231. doi: 10.1172/JCI111309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J. 1987 Oct;1(4):272–281. doi: 10.1096/fasebj.1.4.3308611. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Origin of the rat mesangial phagocyte and its expression of the leukocyte common antigen. Lab Invest. 1984 Nov;51(5):515–523. [PubMed] [Google Scholar]
- Sedor J. R., Carey S. W., Emancipator S. N. Immune complexes bind to cultured rat glomerular mesangial cells to stimulate superoxide release. Evidence for an Fc receptor. J Immunol. 1987 Jun 1;138(11):3751–3757. [PubMed] [Google Scholar]
- Singhal P. C., Ding G. H., DeCandido S., Franki N., Hays R. M., Schlondorff D. Endocytosis by cultured mesangial cells and associated changes in prostaglandin E2 synthesis. Am J Physiol. 1987 Apr;252(4 Pt 2):F627–F634. doi: 10.1152/ajprenal.1987.252.4.F627. [DOI] [PubMed] [Google Scholar]
- Striker G. E., Striker L. J. Glomerular cell culture. Lab Invest. 1985 Aug;53(2):122–131. [PubMed] [Google Scholar]
- Unkeless J. C., Fleit H., Mellman I. S. Structural Aspects and Heterogeneity of Immunoglobulin Fc Receptors. Adv Immunol. 1981;31:247–270. doi: 10.1016/s0065-2776(08)60922-0. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Duque R. E., Sulavik M. C., Johnson K. J. In vitro and in vivo stimulation of rat neutrophils and alveolar macrophages by immune complexes. Production of O-2 and H2O2. Am J Pathol. 1983 Mar;110(3):297–309. [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Ward P. A. Immune complex induced generation of oxygen metabolites by human neutrophils. J Immunol. 1982 Jul;129(1):309–313. [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Prescott S. M. Production of platelet-activating factor by human vascular endothelial cells: evidence for a requirement for specific agonists and modulation by prostacyclin. Circulation. 1985 Oct;72(4):718–727. doi: 10.1161/01.cir.72.4.718. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Prescott S. M. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J Clin Invest. 1985 Dec;76(6):2235–2246. doi: 10.1172/JCI112232. [DOI] [PMC free article] [PubMed] [Google Scholar]




