ABSTRACT
Since its first implementation in patients in 1991, endovascular aneurysm repair for abdominal aortic aneurysms has gained wide acceptance and availability. This development has been fueled in great part by advances in stent-graft design and by a variety of devices for various anatomic features. This commentary will summarize some of these endograft developments and indicate the path forward for novel endoprostheses.
Keywords: AAA, endovascular aneurysm repair (EVAR), stent graft, endograft
Herein we present a review and some commentary on the current and future state of endovascular aneurysm repair (EVAR) devices, for the treatment of abdominal aortic aneurysms (AAAs). The discussion will commence with a brief review of the history of endovascular AAA repair.
PAST
No history of EVAR may start without mention of Dr. Juan Parodi's elective repair in five patients with AAA in 19911 using a custom-made Dacron tube endoprosthesis, inserted transfemorally, and fixed with balloon-expandable stents. Subsequently, bifurcated endografts were described and implanted, initially in 1994.2 And aorto-uni-iliac or aorto-uni-femoral grafts, requiring coincident occlusion of the contralateral common iliac and femoral-femoral bypass grafting, were reported in 19973,4; many still consider this approach the standard or best for the endovascular treatment of ruptured aneurysms.
Over time, EVAR gained traction for use in patients with ruptured aneurysms; this approach was initially reported in 1994 by Yusuf et al.5 The modular design of current endoprostheses was pioneered by the Sydney, Australia group.6
PRESENT
There are presently five endograft platforms, from four companies, which have received U.S. Food and Drug Administration (FDA) approval for clinical use. These devices, their manufacturers, and their dates of FDA approval are listed in Table 1. The largest proximal neck treated by each device is listed in Table 2.
Table 1.
FDA-Approved Devices for EVAR
| Device | Manufacturer | Date of Initial FDA Approval | Stent Material | Graft Material | Delivery System | Distinctive Feature |
|---|---|---|---|---|---|---|
| FDA, U.S. Food and Drug Administration; EVAR, endovascular aneurysm repair; ePTFE, expandable polytetrafluoroethylene. | ||||||
| AneuRx AAAdvantage | Medtronic | 1999 | Nitinol | Woven polyester | Integrated sheath | No anatomic fixation nor pararenal stent |
| Excluder | Gore | 2002 | Nitinol | ePTFE | Separate sheath* | Infrarenal active (hooks) fixation |
| Zenith Flex | Cook | 2003 | Stainless steel | Woven polyester | Integrated sheath | Pararenal uncovered stent with suprarenal active (hooks) fixation |
| Powerlink | Endologix | 2004 | Cobalt chromium alloy | ePTFE | Integrated sheath | Unibody design |
| Talent | Medtronic | 2008 | Nitinol | Woven polyester | Integrated sheath | Pararenal uncovered stent |
In contrast to the other stent grafts listed, the Gore Excluder is delivered through a separate sheath: there is no sheath integrated into the delivery system.
Table 2.
Largest Proximal Neck Treated: Current FDA-Approved Devices
| Device | Largest Neck Treated (mm) | Sheath (OD) to Treat 26-mm Proximal Neck (Fr) |
|---|---|---|
| FDA, U.S. Food and Drug Administration; OD, outer diameter; Fr, French. | ||
| AneuRx AAAdvantage | 26 | 21 |
| Excluder | 26† | 21* |
| Zenith Flex | 32 | 24 |
| Powerlink | 32 | 22 |
| Talent | 32 | 24 |
In contrast to the other stent grafts listed, the Gore Excluder is delivered through a separate sheath: there is no sheath integrated into the delivery system.
The Excluder 31-mm device can treat 27-mm to 29-mm aortic necks, but it is currently available in the United States for investigational use only. The sheath that Gore markets to deliver the 28-mm aortic endoprosthesis is 18-Fr with an OD of 21-Fr.
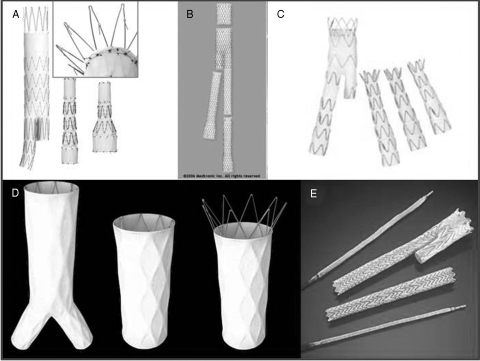
The Cook Zenith Flex device (Bloomington, IN) had long been the only FDA-approved endoprosthesis capable of treating 32-mm proximal aortic necks (the Zenith accomplishes this with a 36-mm graft),7 until the Powerlink 34-mm aortic cuffs and Medtronic Talent were approved in 2008. Its design is remarkable for a pararenal uncovered stent with active fixation (hooks), as demonstrated in the inset to Fig. 1A.
Figure 1.
Currently available Food and Drug Administration–approved stent grafts for treatment of abdominal aortic aneurysm. (A) Zenith. (B) AneuRx. (C) Talent. (D) Powerlink. (E) Excluder.
Medtronic (Minneapolis, MN) currently markets two endografts for infrarenal AAAs. The AneuRx device is marketed in part as the device with the smallest delivery system to treat a 26-mm aortic neck: the AneuRx 28-mm proximal main body comes with in a 21-French (outer diameter) integrated sheath.8 The AneuRx has no active fixation: neither proximal hooks nor the ability to rest on the aortic bifurcation (Fig. 1B). Possibly because of the more complete long-term survival data amassed in studies of this graft, the FDA has issued a series of warnings (most recently in 2008) that late aneurysm-related mortality may be higher in patients treated with the AneuRx device than in those treated with conventional open surgery.9
The Talent device is also manufactured by Medtronic and is the only FDA-approved device whose on-label instructions for use allow treating aortic necks as short as 10 mm.10 The Talent is a modular bifurcated graft, but given the large variety of main body configurations, repair can often be accomplished with a two-piece design. Although the Talent has no active fixation by proximal hooks, it does incorporate a suprarenal uncovered stent (Fig. 1C).
The Powerlink device, from Endologix (Irvine, CA), is the only currently marketed device that is described as “unibody,” as opposed to modular bifurcated.11 (Contrast Fig. 1D with the other devices depicted in Fig. 1.) Among the benefits of this system are: (1) contralateral access is accomplished—usually percutaneously—with a very small-diameter sheath (9-French), and (2) the usually obligatory—and often time-consuming—contralateral gate cannulation is rendered unnecessary by the unibody design.
Recently, Endologix has obtained FDA approval for some new components of its system, including suprarenal extensions (comprising an uncovered stent proximal to an aortic cuff), 34-mm aortic cuffs capable of treating proximal necks up to 32 mm in diameter, and iliac limb extensions with distal diameters of 25 mm capable of treating iliacs up to 23 mm.
Finally, Gore (Flagstaff, AZ) produces the Excluder device for EVAR.12 This device is distinct from all of the above endografts in that it is delivered through standard percutaneous introducer sheaths; there is no sheath incorporated into the delivery system. This approach to stent-graft insertion is compelling in its flexibility and adaptability, especially in the situation of small or otherwise difficult iliofemoral access vessels. Using the Gore device, it is most often possible to design a two-piece repair of an AAA, given the vast variety of diameters and lengths of the integrated main body and ipsilateral limb graft (Fig. 1E). Earlier versions of the Excluder had lower rates of aneurysm sac shrinkage than with other devices; most recently, a “low-permeability” version is manufactured and preliminary results indicate that it is less prone to “endotension” (sac expansion in absence of demonstrable endoleak). Although Gore plans the addition of devices with larger-diameter proximal aortic necks, currently the largest device is 28.5 mm proximally, which is able to treat up to 26-mm necks. This is smaller than other current FDA-approved devices, except the AneuRx endoprosthesis.
One additional EVAR device had been FDA approved: the Ancure device from EVT, a subsidiary of Guidant, was FDA-approved in 1999, but its approval was suspended in 2001 due to nonreporting of many device failures and adverse patient outcomes. Subsequently reapproved, Guidant stopped manufacturing the device in 2003.
Several other devices have undergone FDA-sanctioned trial in the United States. The Edwards Lifepath, a balloon-expandable modular bifurcated graft, suffered from stent fractures of the main body.13 The Cordis Fortron incorporated transrenal fixation but has never come to market.14 Finally, the Trivascular Enovus device was deliverable through a small-diameter sheath because its support structure was in part derived from injectable polymer.15 The Enovus also employed a “gasket” at its proximal seal zone, with the capability to seal in angled or calcified necks, which, in necks with circumferential thrombus, may be augmented by iterations on this device, similar designs, or other mechanisms such as “staples.”16
FUTURE
Required secondary interventions during long-term follow-up of patients having undergone EVAR, to prevent aneurysm-related mortality, partially offset early advantages in recovery and perioperative mortality as compared with open repair.17 Therefore, device design to maximize the durability of EVAR and safely minimize the requirements for follow-up and monitoring will continue to be sought after.
Increasing the applicability of EVAR to patients with difficult anatomic features will likewise require improvements and innovation in device design. The three most common anatomic reasons for exclusion from endovascular repair of AAAs are inappropriate aortic (proximal) neck, iliac artery aneurysms, and inadequate iliac access.18,19
The next frontier of endovascular AAA repair is clearly the continued adoption and development of endografts capable of treating juxtarenal, pararenal, and thoracoabdominal aortic aneurysms.
Therefore, the challenges for the next generation of EVAR devices are: (1) increasing the durability of stent-graft repair, (2) refining and redesigning grafts to allow treatment of today's “difficult” anatomic features, and (3) advancing our abilities to treat aneurysms involving the renal and visceral segments of the aorta.
Future Devices
Several endografts for EVAR are currently on trial in the United States or are awaiting FDA approval (Table 3). The Anaconda device, developed by Vascutek, is currently in the FDA approval process.20,21 It is purported to provide facile contralateral gate cannulation, owing to a unique dual-wire system equipped with magnets, flexible delivery system, and—most remarkably—the capacity to collapse and reposition after the proximal neck (with active fixation hooks) has been deployed. In addition, the proximal ring stent has a saddle-shaped stent, allowing a portion of the proximal device to be placed at and above the renals, without actually covering the ostia.
Table 3.
EVAR Devices Currently in Trial or Limited Use
| Device | Manufacturer | Stent Material | Fabric Material | Delivery System | Distinctive Features |
|---|---|---|---|---|---|
| EVAR, endovascular aneurysm repair; Fr, French. | |||||
| Anaconda | Vascutek | Nitinol | Woven polyester | Integrated sheath | • Repositioning capability |
| • Magnetic guidance for cannulation of contralateral gate | |||||
| Aorfix | Lombard | Nitinol | Woven polyester | Integrated sheath | • Circumferential ring stents and “crumple zones” |
| • May provide flexibility for angulated necks | |||||
| Aptus | Aptus | Nitinol | Woven polyester | Integrated sheath | • Small delivery system (16 Fr) |
| • Endostaples | |||||
| Endurant | Metronic | Nitinol | Woven polyester | Integrated sheath | • Pararenal uncovered stent with suprarenal active (hooks) fixation |
The Aorfix device, from Lombard, features circumferential ring stents with intervening “crumple zones” at its proximal seal zone, in an attempt to allow treatment of more angulated hostile necks.22
The Aptus endoprosthesis incorporates novel proximal neck screws, or “endostaples,” providing fixation of the graft to the transmural aorta. The device requires only a 16-French delivery system. The pivotal trial is currently underway in the United States.
Medtronic has a novel device, called Endurant, which is currently being used in Europe, with upcoming trials in the United States. This device incorporates both the pararenal uncovered stent that the company utilizes in its Talent endoprosthesis and active fixation hooks proximally.
Improvements to Current Devices
The various companies with FDA-approved EVAR devices are all fairly secretive with respect to novel devices, components, or features currently in development. That said, a couple of these improvements are either currently in trial or are widely anticipated. Since 2003, Gore has marketed a 31-mm Excluder device for sealing proximal necks up to 29 mm. This device has been trialed in the United States but is not currently available for regular use. A “next-generation” Cook Zenith device is in development: among its features is a greatly reduced sheath diameter (reportedly, 16-French to 18-French outer diameter).
Fixation
Current approaches to fixation of grafts—to prevent migration, especially of the proximal graft from its implanted juxtarenal position—include: (1) radial force; (2) hooks, anchors, or barbs to embed in the aortic wall; (3) columnar stiffness of the graft with distal anchoring in the iliacs or on the aortic bifurcation (the Powerlink device). Hooks may be positioned in either the infrarenal portion of the graft (the Gore Excluder) or in the pararenal uncovered stent (Zenith and Endurant devices). The Aptus device attempts to address this issue in a novel and unique manner, as discussed previously.
Branched and Fenestrated Endografts
Branched grafts, fenestrated grafts, or some combination thereof may potentially extend EVAR to AAAs with short or “difficult” proximal necks, as well as to juxtarenal and suprarenal aneurysms.23 The first reported experience with fenestrated endografts to treat aortic aneurysms with branch vessel ostia in the anticipated seal zone came in 1996 from Park et al.24 Subsequently, the Cleveland Clinic group has reported their experience with a Cook fenestrated device.25,26
Branched Iliac Grafts
In current practice, common iliac involvement in aneurysmal change (aortoiliac aneurysm) is most often treated with embolization of the ipsilateral internal iliac (except in ruptured AAAs) and coverage of the ostium of that vessel by landing and sealing the stent-graft limb in the external iliac. However, this approach has several disadvantages: it is seldom utilized in cases of bilateral common iliac aneurysms (owing to the high risk of colonic ischemia), it can result in ipsilateral gluteal claudication, and it makes iliac stent-graft limb patency entirely dependent on femoral outflow, which can be compromised in patients with atherosclerosis who have undergone femoral access and repair. Early iterations of devices incorporating branched iliac endografts have been reported but are not currently on trial in the United States.
DISCUSSION
The two most frequently encountered barriers to EVAR are inadequate or inappropriate proximal neck anatomy and inadequate caliber of iliofemoral access vessels. Hence, the abilities of any device, present or future, to accommodate patients with difficult necks or treacherous access vessels are important, even essential. Novel bifurcated endografts and the development of widely available branched and fenestrated grafts will help address these barriers, building upon the remarkable clinical success of the past and current generations of endovascular prostheses for the treatment of AAAs.
REFERENCES
- Parodi J C, Palmaz J C, Barone H D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–499. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- White G H, Yu W, May J, Stephen M S, Waugh R C. A new nonstented balloon-expandable graft for straight or bifurcated endoluminal bypass. J Endovasc Surg. 1994;1:16–24. doi: 10.1583/1074-6218(1994)001<0016:ANNBEG>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ivancev K, Malina M, Lindblad B, et al. Abdominal aortic aneurysms: experience with the Ivancev-Malmo endovascular system for aorto-monoiliac stent-grafts. J Endovasc Surg. 1997;4:242–251. doi: 10.1177/152660289700400303. [DOI] [PubMed] [Google Scholar]
- Ohki T, Veith F J, Sanchez L A, et al. Varying strategies and devices for endovascular repair of abdominal aortic aneurysms. Semin Vasc Surg. 1997;10:242–256. [PubMed] [Google Scholar]
- Yusuf S W, Whitaker S C, Chuter T A, et al. Emergency endovascular repair of leaking aortic aneurysm. Lancet. 1994;344:1645. doi: 10.1016/s0140-6736(94)90443-x. [DOI] [PubMed] [Google Scholar]
- May J, White G H, Yu W, et al. Current comparison of endoluminal versus open repair in the treatment of abdominal aortic aneurysms: analysis of 303 patients by the life table method. J Vasc Surg. 1998;27:213–221. doi: 10.1016/s0741-5214(98)70352-0. [DOI] [PubMed] [Google Scholar]
- Greenberg R K, Chuter T AM, Sternbergh W C, et al. Zenith AAA endovascular graft: intermediate-term results of the US multicenter trial. J Vasc Surg. 2004;39:1209–1218. doi: 10.1016/j.jvs.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Arko F R, Murphy E H. Endovascular aneurysm repair utilizing the AneuRx and Talent stent grafts. Perspect Vasc Surg Endovasc Ther. 2008;20:120–128. doi: 10.1177/1531003508320226. [DOI] [PubMed] [Google Scholar]
- FDA Public Health Notification Updated Data on Mortality Associated with the Medtronic AneuRx® Stent Graft System. Available at: http://www.fda.gov/cdrh/safety/031808-medtronic.html. Accessed March 17, 2008. Available at: http://www.fda.gov/cdrh/safety/031808-medtronic.html
- Criado F J, Fairman R M, Becker G J, et al. Talent LPS AAA stent graft: results of a pivotal clinical trial. J Vasc Surg. 2003;37:709–715. doi: 10.1067/mva.2003.230. [DOI] [PubMed] [Google Scholar]
- Wang G J, Carpenter J P. The Powerlink System for endovascular aortic abdominal aneurysm repair: six year results. J Vasc Surg. 2008;48:535–545. doi: 10.1016/j.jvs.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Tanski W, III, Fillinger M F. Outcomes of original and low-permeability Gore Excluder endoprosthesis for endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2007;45:243–249. doi: 10.1016/j.jvs.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Carpenter J P, Anderson W N, Brewster D C, et al. Multicenter pivotal trial results of the Lifepath System for endovascular aortic aneurysm repair. J Vasc Surg. 2004;39:34–43. doi: 10.1016/j.jvs.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Brener B J, Faries P L, Connelly T, et al. An in situ adjustable endovascular graft for the treatment of abdominal aortic aneurysms. J Vasc Surg. 2002;35:114–119. doi: 10.1067/mva.2002.119748. [DOI] [PubMed] [Google Scholar]
- Katzen B T. ENOVUS stent-graft: early clinical experience. J Endovasc Ther. 2004;11:I22–I23. [Google Scholar]
- Donas K P, Kafetzakis A, Umscheid T, et al. Vascular endostapling: new concept for endovascular fixation of aortic stent-grafts. J Endovasc Ther. 2008;15:499–503. doi: 10.1583/08-2467.1. [DOI] [PubMed] [Google Scholar]
- Carpenter J P, Baum R A, Barker C F, et al. Durability of benefits of endovascular versus conventional abdominal aortic aneurysm repair. J Vasc Surg. 2002;35:222–228. doi: 10.1067/mva.2002.120034. [DOI] [PubMed] [Google Scholar]
- Elkouri S, Martelli E, Gloviczki P, et al. Most patients with abdominal aortic aneurysm are not suitable for endovascular repair using currently approved bifurcated stent grafts. Vasc Endovascular Surg. 2004;38:401–412. doi: 10.1177/153857440403800502. [DOI] [PubMed] [Google Scholar]
- Moise M A, Woo E Y, Velazquez O C, et al. Barriers to endovascular aortic aneurysm repair: past experience and implications for future device development. Vasc Endovascular Surg. 2006;40:197–203. doi: 10.1177/153857440604000304. [DOI] [PubMed] [Google Scholar]
- Saratzis N, Melas N, Saratzis A, et al. Anaconda aortic stent-graft: single-center experience of a new commercially available device for abdominal aortic aneurysms. J Endovasc Ther. 2008;15:33–41. doi: 10.1583/07-2277.1. [DOI] [PubMed] [Google Scholar]
- Freyrie A, Gargiulo M, Rossi C, et al. Preliminary results of Anaconda aortic endografts: a single center study. Eur J Vasc Endovasc Surg. 2007;34:693–698. doi: 10.1016/j.ejvs.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Albertini J N, Perdikides T, Soong C V, et al. Endovascular repair of abdominal aortic aneurysms in patients with severe angulation of the proximal neck using a flexible stent-graft: European Multicenter Experience. J Cardiovasc Surg (Torino) 2006;47:245–250. [PubMed] [Google Scholar]
- Chuter T A. Fenestrated and branched stent-grafts for thoracoabdominal, pararenal and juxtarenal aortic aneurysm repair. Semin Vasc Surg. 2007;20:90–96. doi: 10.1053/j.semvascsurg.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Park J H, Chung J W, Choo I W, et al. Fenestrated stent grafts for preserving visceral arterial branches in the treatment of abdominal aortic aneurysms: preliminary experience. J Vasc Interv Radiol. 1996;7:819–823. doi: 10.1016/s1051-0443(96)70854-0. [DOI] [PubMed] [Google Scholar]
- Greenberg R K, Haulon S, O'Neill S, et al. Primary endovascular repair of juxtarenal aneurysms with fenestrated endovascular grafting. Eur J Vasc Endovasc Surg. 2004;27:484–491. doi: 10.1016/j.ejvs.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Haddad F, Greenberg R K, Walker E, et al. Fenestrated endovascular grafting: the renal side of the story. J Vasc Surg. 2005;41:181–190. doi: 10.1016/j.jvs.2004.11.025. [DOI] [PubMed] [Google Scholar]