Abstract
Major depressive disorder (MDD) is associated with action monitoring dysfunction, particularly disrupted error processing. Whether such dysregulation is further modulated by task incentives is largely unknown. The goal of this study was to investigate possible dysfunctions in error processing in MDD as a function of varying task incentives and clinical profile. To this end, we recorded the error-related negativity (ERN) and error positivity (Pe) in 18 MDD subjects and 18 healthy controls during a Stroop task that intermixed no-incentive and reward trials. Relative to controls, MDD subjects showed (1) larger ERN irrespective of task incentives, and (2) reduced Pe during reward (but not no-incentive) trials. Moreover, among MDD subjects, Pe amplitudes were negatively correlated with depression severity and clinical symptoms. The present findings highlight distinct effects of task incentives on electrophysiological components of error processing and are interpreted within current theories of action monitoring and incentive processing in depression.
Keywords: ERN, Pe, Depression, Reward, Anhedonia, Action Monitoring
Introduction
One aspect of cognition that has attracted considerable interest is the ability to adjust performance based on shifting incentives and the consequences of previous events (action monitoring). This capacity allows us to coordinate actions in a manner that maximizes the likelihood of achieving intended goals, even following unexpected changes in the environment. Major depressive disorder (MDD) is characterized by action monitoring dysfunction (particularly reduced behavioral performance in situations requiring adaptive strategy shifts; Beats, Sahakian, & Levy, 1996; Elliott et al., 1996; Holmes & Pizzagalli, 2008) and decreased approach-related behavior (for a review, see Pizzagalli, Dillon, Bogdan, & Holmes, in press). Thus, impairments in action monitoring might be partially explained by abnormalities in incentive processing. Providing initial support for this hypothesis, among healthy controls, action monitoring has been found to be modulated by motivational/emotional context and individual differences in reward sensitivity (Boksem, Tops, Kostermans, & De Cremer, 2008; Holmes & Pizzagalli, 2007; Luu, Tucker, Derryberry, Reed, & Poulsen, 2003).
A growing literature suggests that dysregulated activity within structures critically implicated in action monitoring and incentive processing, including the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC), could contribute to behavioral impairments in MDD (e.g., Alexopoulos et al., 2005; Forbes et al., 2006; Siegle, Thompson, Carter, Steinhauer, & Thase, 2007). Specifically, dysfunctional ACC activity in MDD has been associated with reduced performance following perceived failure (errors) or negative feedback (Holmes & Pizzagalli, 2008; Tucker, Luu, Frishkoff, Quiring, & Poulsen, 2003) as well as reduced responsiveness to positive reinforcement (e.g., monetary incentives; Forbes et al., 2006). Altogether, prior behavioral and neuroimaging findings raise the possibility that the action monitoring system could be differentially modulated by shifts in task-relevant incentives in healthy controls and MDD subjects.
Due to the rapid nature of neural processes implicated in action monitoring, research in this area has relied on electrophysiological markers of performance monitoring. Capitalizing on the temporal resolution of event-related potential (ERP) recordings, studies have primarily focused on two response-related waveforms, the error-related negativity (ERN) and error positivity (Pe), which might represent partially dissociable components of error processing (e.g., Falkenstein et al., 2000; Holroyd & Coles, 2002; Overbeek, Nieuwenhuis, & Ridderinkhof, 2005; van Veen & Carter, 2002).
ERN
Occurring 50–150 ms following an incorrect response, and elicited even when participants are unaware of having committed a mistake (e.g., Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001; Shalgi, Barkan, & Deouell, 2009), the ERN is a negative deflection with frontocentral scalp distribution believed to index the automatic detection of events (e.g., errors) that require increased cognitive control (e.g., Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993). While the function of the ERN remains debated, recent findings suggest that, in addition to automatic error detection, it may reflect a more general estimation of the motivational value of ongoing events (e.g., Boksem, Tops, Kostermans, & De Cremer, 2008; Hajcak, Holroyd, Moser, & Simons, 2005; Luu, Collins, & Tucker, 2000).
Consistent with this argument, among healthy controls, ERN amplitude is larger (i.e., more negative) to errors committed when monetary incentives are available (e.g., Ganushchak & Schiller, 2008; Gehring & Willoughby, 2002; Hajcak, Moser, Yeung, & Simons, 2005). Along similar lines, in a recent study using an Eriksen Flanker task under varying incentive conditions, students high on reward sensitivity showed heightened ERN when failing to earn rewards due to errors, but not when they were punished for committing mistakes (Boksem et al., 2008). In contrast, subjects with high punishment sensitivity showed largest ERN under the punishment condition (Boksem et al., 2008). Collectively, these findings suggest that the ERN is sensitive to the motivational significance of errors (see Olvet & Hajcak, 2008 for a recent review).
ERN findings in MDD have been inconsistent. Potentiated ERN has been observed in moderately depressed subjects during experimental tasks devoid of incentive manipulations in some (e.g., Alexopoulos et al., 2007; Chiu & Deldin, 2007; Holmes & Pizzagalli, 2008) but not all (Ruchsow et al., 2004; Ruchsow et al., 2006) studies as well as in tasks involving possible penalties (Chiu & Deldin, 2007), whereas no differences between MDD and control subjects emerged within the context of reward (Chiu & Deldin, 2007). Conversely, in severely depressed samples, reduced ERN amplitudes following errors have emerged under no-incentive condition, a finding generally attributed to the presence of apathy and/or psychomotor slowing in more severe cases of depression (Schrijvers et al., 2008; Schrijvers et al., 2009b). Accordingly, under baseline (no-incentive) conditions, MDD subjects appear to be characterized by exaggerated automatic responses to perceived failures. However, when errors occur within the context of rewards, group differences may be attenuated due to the fact that healthy controls generate relatively larger ERN during reward relative to no-incentive conditions (Ganushchak & Schiller, 2008; Gehring & Willoughby, 2002; Hajcak et al., 2005), whereas MDD subjects may lack this potentiation due anhedonia and blunted reward responsiveness (e.g., Forbes et al., 2009; Pizzagalli et al., 2009a; Pizzagalli et al., 2009b). Based on these arguments and prior studies (Chiu & Deldin, 2007; Holmes & Pizzagalli, 2008), we hypothesized that, relative to controls, MDD subjects would show larger ERN to errors, particularly under no-incentive conditions.
Pe
The Pe is a positive voltage deflection peaking approximately 200 to 400 milliseconds after error commission (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000). Although the functional significance of the Pe remains to be delineated, it has been hypothesized to reflect error awareness, affective appraisal of errors, or processes implicated in post-error behavioral adjustments (e.g., Hajcak, McDonald, & Simons, 2003; Nieuwenhuis et al., 2001; Shalgi et al., 2009; for review see Falkenstein et al., 2000; Overbeek et al., 2005). In addition, similarities between the Pe and P3b component have led to the hypothesis that the Pe might be linked to the motivational significance of errors (Overbeek et al., 2005). In line with this argument, Pe amplitude has been found to be positively correlated with individual differences in reward sensitivity (e.g., Boksem, Tops, Wester, Meijman, & Lorist, 2006; Boksem et al., 2008), and larger when errors are salient (e.g., Leuthold & Sommer, 1999). These findings have been interpreted as suggesting that, following a mistake in performance, the Pe might reflect a mechanism to prevent the occurrence of subsequent errors, maximizing future rewards (i.e., approach motivation/reward seeking; Boksem et al., 2006).
Pe findings in depression have been equally inconsistent. Specifically, whereas reduced Pe has been described in severely depressed individuals (Alexopoulos et al., 2007; Schrijvers et al., 2008; Schrijvers et al., 2009a), findings in moderately depressed subjects have not emerged (Chiu & Deldin, 2007; Compton et al., 2008; Holmes & Pizzagalli, 2008). Prior null findings in participants with moderate levels of depressive symptoms were interpreted as suggesting that disruption in later stages of error processing might emerge only in samples of treatment-resistant patients and/or geriatric patients with depression (Holmes & Pizzagalli 2008). However, it is important to emphasize that null findings have been observed either in experimental tasks without manipulations of incentive motivation (Compton et al., 2008; Holmes & Pizzagalli, 2008) or tasks involving separate blocks of reward and no-incentive trials (Chiu & Deldin, 2007). Because reduced Pe in severe depression has been attributed to anhedonia and apathy (Schrijvers et al., 2009a), and in light of findings in healthy controls implicating the Pe in processing the motivational significance of errors (Overbeek et al., 2005), we reasoned that Pe differences might emerge in tasks manipulating incentive motivation on a trial-by-trial basis. Based on these arguments and findings highlighting reduced reward responsiveness in depression (e.g., Pizzagalli et al., 2009a; Pizzagalli et al., 2009b), we hypothesized that moderately depressed subjects would display reduced Pe, particularly within the context of reward trials.
In sum, the goal of the present study was to investigate putative dysfunctions in error processing in MDD as a function of varying task incentives and clinical profile. To this end, 128-channel ERPs were recorded as healthy controls and unmedicated, moderately depressed MDD subjects performed a Stroop task under different incentive conditions (no-incentive vs. reward). In addition to testing hypotheses concerning group differences in ERN and Pe as a function of task incentives, correlational analyses were performed to investigate whether these error-related ERP components were further modulated by depression severity and anhedonia. In light of inconsistent findings in the literature (e.g., Alexopoulos et al., 2007; Holmes and Pizzagalli, 2008; Schrijvers et al., 2009b), these latter analyses were considered exploratory.
Methods
Participants
Eighteen participants with MDD and 18 controls were recruited from the community through flyers and Internet postings. General inclusion criteria included right-handedness (Chapman & Chapman, 1987), age between 18 and 55 years, and normal or corrected-to-normal vision. Participants were excluded if they reported neurological disorders; a history of electroconvulsive therapy, seizures, or head injury resulting in loss of consciousness; or past/current substance abuse or dependence (with the exception of past alcohol and marijuana abuse > 1 year ago). None of the participants had prior research experience in our laboratory; consequently, the current sample has not been included in any prior publications (Holmes and Pizzagalli, 2008).
Depressed subjects met DSM-IV diagnostic criteria for MDD (American Psychiatric Association, 1994), as assessed by the Structured Clinical Interview for the DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2007). MDD subjects were excluded if they reported current or past psychotic symptomatology or met criteria for any current Axis I diagnosis other than MDD (with the exception of simple phobia, n = 2), and if they received psychotropic medication within the last 2 weeks (4 weeks for neuroleptics and benzodiazepines, 6 weeks for fluoxetine, and 6 months for dopaminergic drugs). Healthy controls were excluded if they reported current or past psychopathology (as assessed by the SCID) or psychological treatment. Three participants performed at or worse than chance levels in the behavioral task and their data were excluded from the analyses (MDD: n = 2, controls: n = 1).
To investigate possible relationships between ERN/Pe amplitudes and clinical symptoms, the Beck Depression Inventory-II score (BDI-II; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al., 1995) was administered. The MASQ assesses anxiety-specific symptoms (anxious arousal, AA), depression-specific symptoms (anhedonic depression, AD), and general distress (general distress-anxious symptoms, GDA; general distress depressive symptoms, GDD).
Procedure
The study consisted of two separate sessions. In the first session, informed written consent was collected after the study was described. Subsequently, a SCID was administered by a masters-level interviewer to confirm study eligibility. To evaluate diagnostic reliability, audiotapes from 18 SCID interviews were randomly selected and independently rated by a second interviewer (κ = 1.00). Within one week of establishing study eligibility, participants were invited for the second session, during which they completed self-report questionnaires (BDI, MASQ) and performed a modified version of the Stroop task while 128-channel EEG data were collected. After the task, participants were fully debriefed and received $32 for their participation. The Committee on the Use of Human Subjects at Harvard University approved the protocol.
Apparatus
128-channel EEG data were collected within a sound- and electrically-shielded room using the Geodesic Sensor Net system (Electrical Geodesic, Inc, OR). Data were collected using a 250-Hz sampling rate (bandwidth: 0.01–100 Hz) and the vertex electrode (Cz) as the recording reference. Impedances were kept below 50 KΩ. Eprime software (Psychology Software Tools, Inc., Pittsburgh, PA) was used to present the experiment and interface with the EEG NetStation acquisition software.
Task
All participants completed a modified version of the Stroop task. The stimuli consisted of three words (RED, GREEN, and BLUE) printed in one of three ink colors (red, green, and blue). Each probe could be either congruent (e.g., the word BLUE printed in blue ink) or incongruent (e.g., the word RED printed in green ink). Responses were made using the index, middle, and ring fingers of the right hand. Participants were instructed to press the button, identified through colored stickers, corresponding to the ink color of each stimulus as quickly and as accurately as possible.
Prior to the task, participants completed two 24-trial practice blocks. Reaction times from the second practice block were used to calculate an individual window for early and late responses (see below). During practice, each trial began with the presentation of a fixation cross (250 ms), followed by a Stroop probe (150 ms), and a jittered inter-trial interval (ITI; 1850–1950 ms). Upon completing the practice, participants were informed that, based on their task performance, they would have the opportunity to earn $20 in addition to their base reimbursement ($10/hour). Unlike a prior study that featured separate blocks with and without incentives (Chiu & Deldin 2007), the present design incorporated trial-to-trial shifts in task-relevant incentives allowing us to compare reward and no-incentive conditions while minimizing potential confounds inherent in block designs (e.g., habituation, anticipation, set/strategy effects). Accordingly, participants were instructed that a visual cue signaling either the possibility of a rewarding outcome (+$) or no monetary incentive (0$) would be presented at the beginning of each trial. Participants were also told that, in order to maximize their earnings, they should respond as quickly and accurately as possible and that each trial would end with a feedback informing them about the outcome.
The behavioral task consisted of six blocks, each involving 100 trials. In each block, 50% of the trials were reward and no-incentive trials, respectively. Within each block, 36% of the stimuli were incongruent (64 congruent and 36 incongruent trials/block), evenly distributed across the reward/no-incentive trials. Stimuli were presented using a pseudorandomized sequence, in which no more than three repetitions of incongruent/congruent trials, a given word/color, or a reward/no-incentive cue type were allowed. Thus, each trial involved a cue manipulation and task-relevant feedback. Experimental trials began with the presentation of a cue (1250 ms), which was followed by a jittered inter-stimulus interval (ISI; 950–1050 ms), a Stroop probe (150 ms), a second jittered ISI (1150–1250 ms), the feedback (200 ms), and a variable inter-trial interval (950–1500 ms).
For each trial, correct responses made within the individually calculated response window [85% of each participant’s mean reaction time (RT) from the second practice block] were followed by positive feedback (a schematic smiling face). If participants responded incorrectly, or correctly but outside the response window, they were presented with negative feedback (a schematic frowning face). Participants were instructed that, for a reward cue trial, positive feedback indicated that they successfully earned money for that trial, whereas negative feedback meant that they failed to earn additional money. For the no-incentive trials participants were instructed that neither the positive nor negative feedback would impact their earnings. Participants did not receive feedback about their cumulative earnings during the task. To account for potential performance drifts over time, the response window was recalculated at the middle and end of each behavioral block.
Data reduction
Behavioral data
As noted above, participants who performed at or worse than chance levels were excluded from the analyses (MDD: n = 2, control: n = 1). For the remaining MDD (n = 16) and control (n = 17) subjects, trials without a response or with RT lower than 150 ms or higher than 1000 ms were excluded. Additionally, to reduce the potential effect of outliers, trials with RTs beyond the mean ± 3 standard deviations (following ln transformation) were excluded from all analyses [total # of excluded trials: MDD: mean = 1.63, SD = 3.10; control: 0.77±1.20; t(31) = 1.04, p = 0.31].
ERP data
Data were analyzed in BrainVision (Brain Products GmbH, Gilching, Germany). First, an independent component analysis was utilized to remove activity associated with blinks, eye movements, and other artifacts (Jung et al., 2000). Channels with corrupted signal were individually replaced through spatially weighted linear interpolations. Semi-automatic artifact detection was performed to identify remaining artifacts (maximal amplitude: ±75 µV; within-segment absolute amplitude difference: 150 µV; gradients: 50 µV). The ERP analyses focused on the ERN, its complement for correct responses [correct-response negativity (CRN)], and the Pe. Response-locked ERPs were computed 200 ms prior to and 924 ms following a response. Mirroring the behavioral data analyses, ERPs were computed only for trials in which participants made a response. Data were then band-pass filtered (0–30 Hz, 12 dB/octave), baseline-corrected (−200 ms to −100 ms pre-response onset), and re-derived to an average reference. Grandmean ERP waveforms were calculated by averaging data across conditions and groups.
ERN/CRN
Amplitudes for the ERN and CRN were calculated separately following responses for the reward and no-incentive trials at midline electrode sites (Fz, FCz, and Cz), where the ERN/CRN are largest (e.g., Falkenstein et al., 1991; Gehring et al., 1993; Holmes & Pizzagalli, 2008). Following prior studies, and to minimize the effects of possible slow-wave components, ERN/CRN amplitudes were calculated as the difference between the most negative peak within the time window 0 to 150 ms after incorrect and correct responses and the preceding positive peak −80 prior to and 80 ms following the response (e.g., Schrijvers et al., 2008; Ullsperger & Von Cramon, 2006).
Pe
For each participant and condition (reward and no-incentive), the amplitude of the Pe following incorrect responses was defined as the most positive peak 170–500 ms following an incorrect response at electrodes CPz and Pz (e.g., Schrijvers et al., 2008; Ullsperger et al., 2006).
The cortical regions implicated in adaptive responding to errors partially overlap with those associated with response conflict (Ullsperger & Von Cramon, 2001). To avoid confounding these effects, ERN/CRN and Pe waveforms are traditionally scored only for incongruent trial errors, or while simultaneously considering errors in incongruent and congruent trials (e.g., Holmes & Pizzagalli, 2008; Schrijvers et al., 2008). However, due to the limited number of errors in the congruent condition, Condition (reward, no-incentive) × Trial Type (congruent, incongruent) analyses were not possible. Accordingly, analyses examined group differences in ERN/CRN and Pe amplitudes/latencies during the reward and no-incentive conditions (i.e., collapsing across incongruent and congruent trials). Three MDD subjects were lost due to insufficient numbers of artifact-free error responses (≤ 10 errors per condition). The remaining MDD (n = 13) and control (n = 17) subjects did not differ with respect to demographic variables (ps > 0.21) or the number of available segments [reward error: 25.62±18.09 vs. 25.41±11.68; no-incentive error: 27.23±22.21 vs. 27.18±12.06; ts(28) < 0.037, ps > 0.97].
Statistical Analyses
Exploratory analyses revealed no significant effects of gender or ethnicity; therefore, these variables were not further considered.
Behavioral data
Behavioral analyses focused on group differences in overall RT and accuracy as well as incongruency (Stroop) effects. Increased Stroop effect scores [RTIncongruent trials − RTCongruent trials] and [AccuracyCongruent trials − AccuracyIncongruent trials] are indicative of impairments in cognitive control. For accuracy and RT scores, separate mixed 2 × 2 × 2 analyses of variance (ANOVA) with Group (MDD subjects, controls) as between-subject factor and Trial Type (incongruent, congruent) and Condition (reward, no-incentive) as repeated measures were conducted. Due to the inclusion of incentive cues and task-relevant feedback as well as relatively long delay between an error and a subsequent response (~4 seconds), post-error and post-conflict behaviors were not examined.
ERN
Preliminary ANOVA analyses revealed no ERN, CRN, or Pe latency differences across groups or conditions. Thus, ANOVA focused on amplitudes. For the ERN/CRN analyses, a mixed 2 × 2 × 2 × 3 ANOVA with Group (MDD subjects, controls), Condition (reward, no-incentive), Response (correct, incorrect) and Site (Fz, FCz, Cz) as factors was run (i.e., collapsing across responses for incongruent and congruent stimuli). When applicable, the Greenhouse–Geisser correction was applied (adjusted p- and epsilon (ε) values are reported).
Pe
For the Pe, a 2 × 2 × 2 ANOVA with Group (MDD subjects, controls), Condition (reward, no-incentive), and Site (CPz, Pz) was performed. Throughout the analyses, effect sizes are reported in the form of partial eta squared (η2) values. Post hoc Newman–Keuls tests were performed in case of significant ANOVA findings. For the sake of brevity, only ERP effects involving Group and Condition are presented in detail (other findings are available upon request).
Results
Demographics and self-report data
The demographic and self-report data are summarized in Table 1. The MDD and control groups did not differ with respect to gender [χ2(1) = 1.56, p = 0.21], age [t(31) = 0.41, p = 0.69], ethnicity [χ2(1) = 0.17, p = 0.68], or education [t(31) = 0.79, p = 0.44]. As expected, compared to controls, the unmedicated MDD subjects reported significantly higher depressive symptoms [t(31) = 11.28, p < 0.001], with BDI scores in the moderate range (mean: 26.75, SD: 9.07). Relative to controls, MDD subjects also reported significantly higher scores on each of the four MASQ subscales [all t(31) > 2.93, all ps < 0.005].
Table 1.
Summary of demographic and self-report data
| MDD Subjects (n = 18) |
Control Subjects (n = 18) |
||||
|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | p-value |
| Age | 36.44 | 13.06 | 34.82 | 9.39 | n.s. |
| Years of Education | 16.06 | 2.41 | 15.47 | 1.88 | n.s. |
| Percent Female | 68.8% | n/a | 47.1% | n/a | n.s. |
| Percent Caucasian | 87.5% | n/a | 82.4% | n/a | n.s. |
| BDI-II | 26.75 | 9.07 | 1.00 | 1.12 | < 0.001 |
| MASQ AA | 21.44 | 4.63 | 17.94 | 1.20 | < 0.005 |
| MASQ AD | 86.69 | 10.44 | 48.91 | 7.76 | < 0.001 |
| MASQ GDA | 20.44 | 5.25 | 12.88 | 1.87 | < 0.001 |
| MASQ GDD | 37.88 | 10.14 | 13.49 | 1.68 | < 0.001 |
Note–BDI = Beck Depression Inventory-II; MASQ = Mood and Anxiety Symptom Questionnaire; AA = Anxious Arousal; AD = Anhedonic Depression; GDA = General Distress Anxious Symptoms; GDD = General Distress Depressive Symptoms; n.s. = non-significant
Behavioral data
Table 2 summarizes accuracy and RT data across the various conditions for the two groups. For accuracy, the main effect of Trial Type was significant [F(1, 31) = 44.51, p < 0.001; partial η2 = 0.60], due to the expected higher accuracy for congruent (0.94±0.042) relative to incongruent (0.87±0.085) trials. For RT, the main effect of Trial Type was also significant [F(1, 31) = 47.80, p < 0.001; partial η2 = 0.61], due to the expected slower RT for incongruent (509.08±98.60 ms) relative to congruent (449.53±83.86 ms) trials. Additionally, a main effect of Condition emerged [F(1, 31) = 20.70, p < 0.001; partial η2 = 0.40], driven by quicker RT for reward (472.89±79.32 ms) relative to no-incentive (485.72±80.24 ms) trials, indicating that our reward manipulation was successful. Contrary to our hypotheses, no effects involving Group emerged for either accuracy or RT data (all Fs < 2.43, all ps > 0.13).
Table 2.
Summary of (A) accuracy and (B) reaction time during the Stoop task
| MDD Subjects (n = 16) |
Control Subjects (n = 17) |
p-value | |
|---|---|---|---|
| (A) Accuracy | |||
| Incongruent no-incentive | 0.88±0.11 | 0.85±0.07 | 0.34 |
| Congruent no-incentive | 0.94±0.06 | 0.94±0.03 | 0.86 |
| Incongruent reward | 0.89±0.09 | 0.87±0.08 | 0.34 |
| Congruent reward | 0.94±0.05 | 0.94±0.03 | 0.87 |
| (B) Reaction time | |||
| Incongruent no-incentive | 532.80±109.00 | 497.34±86.20 | 0.31 |
| Congruent no-incentive | 467.71±65.45 | 445.02±67.87 | 0.34 |
| Incongruent reward | 517.16±109.25 | 489.02±92.46 | 0.43 |
| Congruent reward | 451.06±62.99 | 434.31±62.35 | 0.45 |
ERN/CRN
ERN/CRN (no-incentive vs. reward trials, collapsed across incongruent and congruent trials)
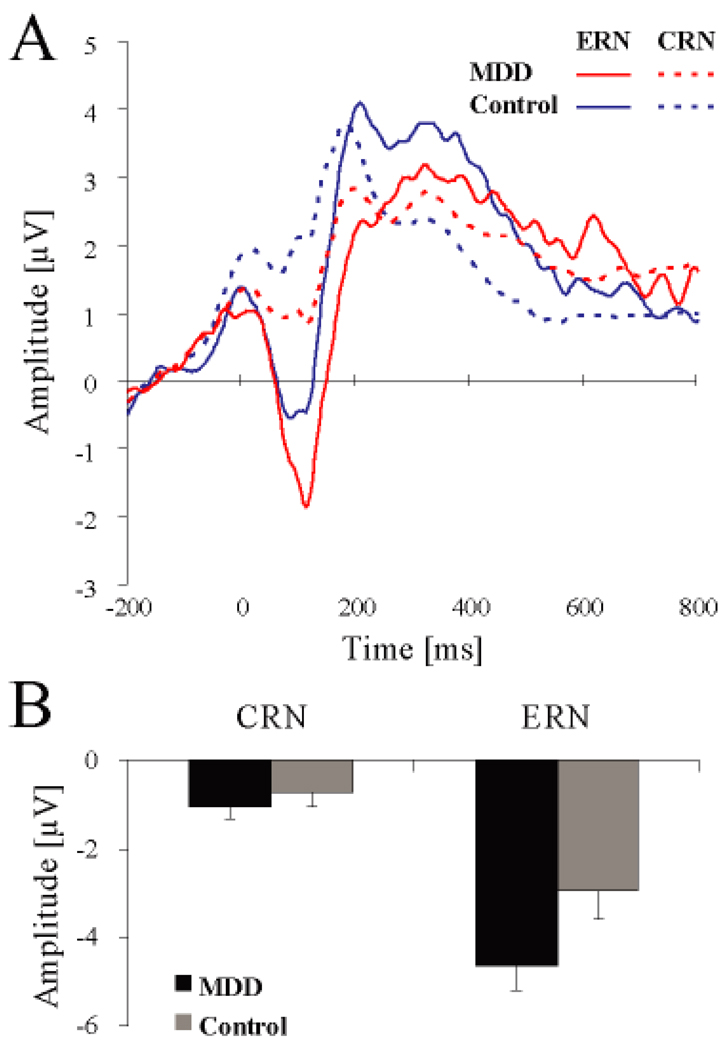
Tables 3A and 4A summarize significant effects emerging from the Group × Condition × Response × Site ANOVA on the ERN and CRN amplitudes. The main effect of Site was significant [F(2, 56) = 29.57, p < 0.001; partial η2 = 0.51], driven by more negative waveforms at Fz (−2.95±1.58µV) and FCz (−2.59±1.83µV) relative to Cz (−1.47±1.78µV; both ps < 0.001) as well as at Fz relative to FCz (p < 0.04). As expected, the main effect of Response was significant [F(1, 28) = 72.17, p < 0.001; partial η2 = 0.72], due to more negative amplitudes for the ERN (−3.79±2.42 µV) relative to CRN (−0.89±0.11 µV). Of primary importance, a significant Group × Response interaction emerged [F(1, 28) = 4.22, p = 0.049; partial η2 = 0.13], indicating that groups differed significantly in their relative potentiation to error responses; the main effect of Group was only trending [F(1, 28) = 3.07, p = 0.091; partial η2 = 0.10]. Follow-up tests revealed that this interaction was driven by increased ERN amplitudes for the MDD (−4.65±2.86 µV) relative to control (−2.94±1.77 µV; p < 0.053) subjects (Figure 1). Importantly, no significant group differences emerged when considering CRN amplitude (MDD: −1.04±0.13; control: −0.76±0.09 p > 0.47). Additionally, both groups displayed more negative amplitudes for the ERN, relative to the CRN (ps < 0.001). No other effects involving either Group or Condition approached significance (all Fs < 2.47, ps > 0.12).
Table 3.
Summary of significant ANOVA effects for (A) ERN, CRN, and (B) Pe amplitudes
| Contrast | F | d.f | p-value | Partial η2 |
|---|---|---|---|---|
| (A) ERN/CRNa | ||||
| Site | 29.57 | 2,56 | < 0.001 | 0.51 |
| Response | 72.17 | 1,28 | < 0.001 | 0.72 |
| Group | 3.07 | 1,18 | 0.091 | 0.10 |
| Group × Response | 4.22 | 1,28 | 0.049 | 0.13 |
| (B) Peb | ||||
| Site | 17.85 | 1,28 | < 0.001 | 0.39 |
| Group × Condition | 4.52 | 1,28 | 0.043 | 0.14 |
Note—
Effects emerging from Group × Condition (no-incentive, reward trials) × Response (correct, incorrect) × Site ANOVAs (collapsed across incongruent and congruent trials).
Effects emerging from Group × Condition (no-incentive, reward trials) × Site ANOVAs (collapsed across incongruent and congruent trials).
Table 4.
Mean (and SD) (A) ERN, CRN, and (B) Pe amplitude for MDD and control subjects (averaged across electrodes of interest).
| MDD Subjects | Control Subjects | p-value | |
|---|---|---|---|
| (A) ERN/CRNa | |||
| ERN | −4.65±2.86 | −2.94±1.77 | 0.053 |
| CRN | −1.04±0.13 | −0.74±0.09 | n.s. |
| (B) Peb | |||
| No-incentive | 2.75±3.76 | 3.23±3.47 | n.s. |
| Reward | 2.07±3.65 | 4.37±4.47 | < 0.004 |
Note—
Averaged across reward and no-incentive feedback as well as incongruent and congruent trials (MDD subjects: n = 13; control subjects: n = 17).
Averaged across incongruent and congruent trials (MDD subjects: n = 13; control subjects: n = 17).
n.s. = non-significant
Figure 1.
(A) Response-locked waveforms for MDD (n = 13; red line) and control (n = 17; blue line) subjects. To reflect the significant Group × Response interaction and to highlight the error-related negativity (ERN), the waveforms were averaged across congruent and incongruent trials, no-incentive and reward trials, and Fz, FCz, Cz. (B) Mean (and S.E.) amplitude scores for the ERN and the correct-response negativity (CRN) averaged across Fz, FCz, Cz for the MDD (black color) and control (grey color) subjects
Contrary to prior studies (Ganushchak & Schiller, 2008; Gehring & Willoughby, 2002; Hajcak et al., 2005), the ERN did not differ between the reward and no-incentive conditions for control subjects (p = 0.17). Similar null findings emerged for subjects MDD (p = 0.89).
Pe
Pe (no-incentive vs. reward trials, collapsed across incongruent and congruent trials)
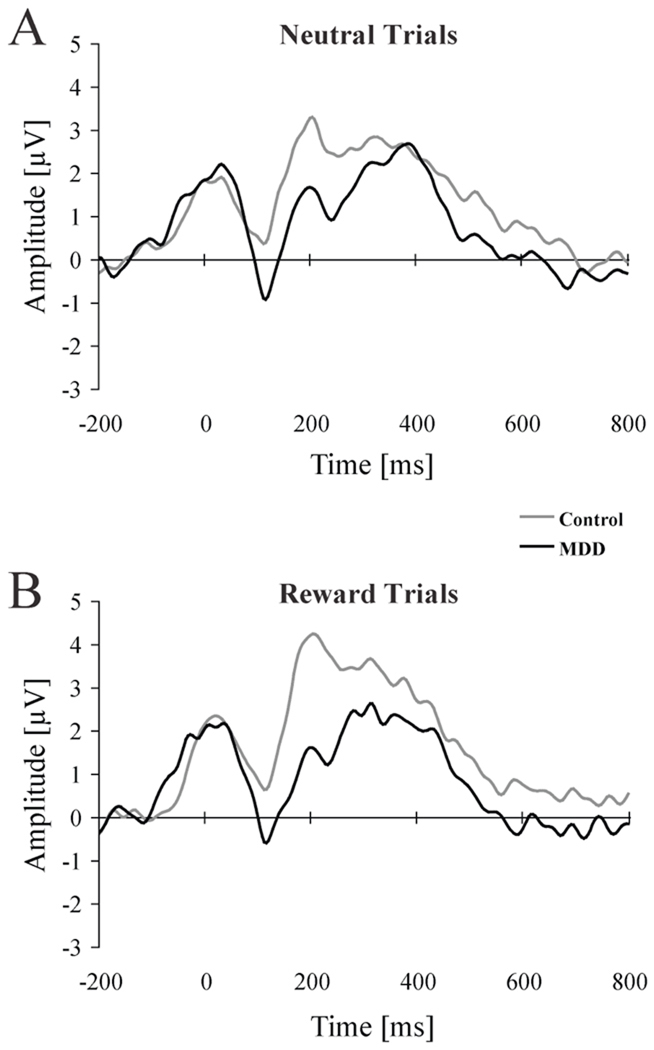
Significant effects emerging from the Group × Condition × Site ANOVA on Pe amplitudes are summarized in Table 3B and 4B. There was a main effect of Site [F(1, 28) = 17.85, p < 0.001; partial η2 = 0.39], due to increased Pe amplitude for CPz (4.08±3.84 µV) relative to Pz (2.31± 3.92 µV). Additionally, and of primary importance to our hypotheses, the Group × Condition interaction was significant [F(1, 28) = 4.52, p < 0.043; partial η2 = 0.14]. Follow-up tests indicated that, while groups did not differ in their Pe amplitudes during no-incentive trials (controls: 3.23±3.47 µV; MDD: 2.75±3.76 µV; p = 0.42; Figure 2A), controls had significantly larger Pe amplitude relative to MDD subjects for the reward condition (4.37±4.47 µV vs. 2.07±3.65 µV; p < 0.004; Figure 2B). In addition, controls displayed a trend for increased Pe for reward relative to no-incentive trials (p < 0.07), whereas MDD subjects did not display any Pe modulation across conditions (p = 0.27).
Figure 2.
Response-locked waveforms for MDD (n = 13; black line) and control (n = 7; gray line) subjects for (A) no-incentive and (B) reward trials. The waveform was averaged across congruent and incongruent trials and across CPz and Pz to highlight the error-positivity (Pe).
Correlation between self-report and ERN/Pe amplitudes (MDD subjects only)
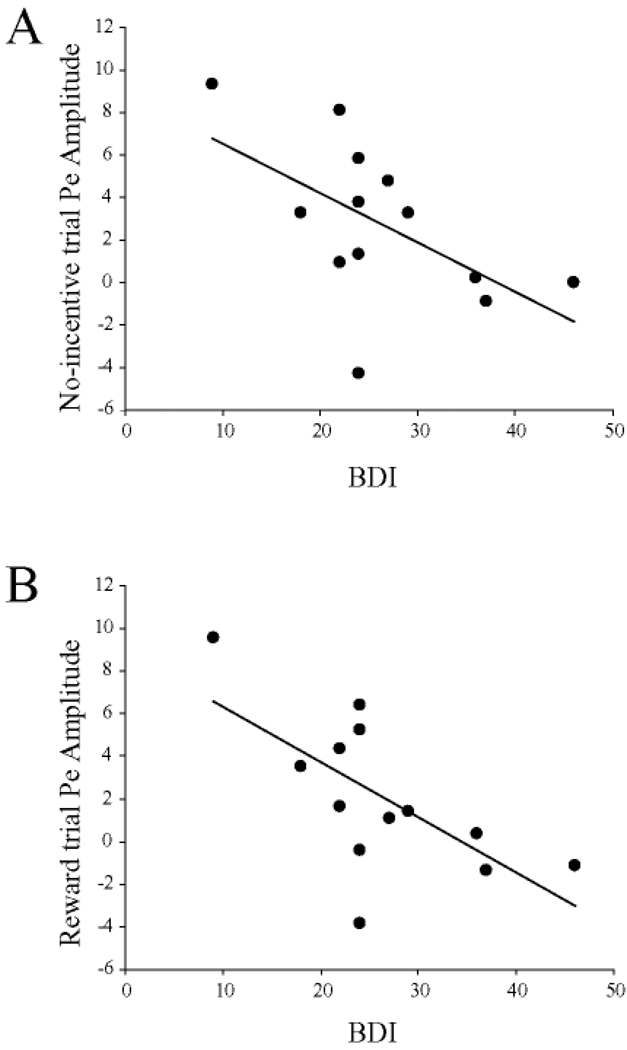
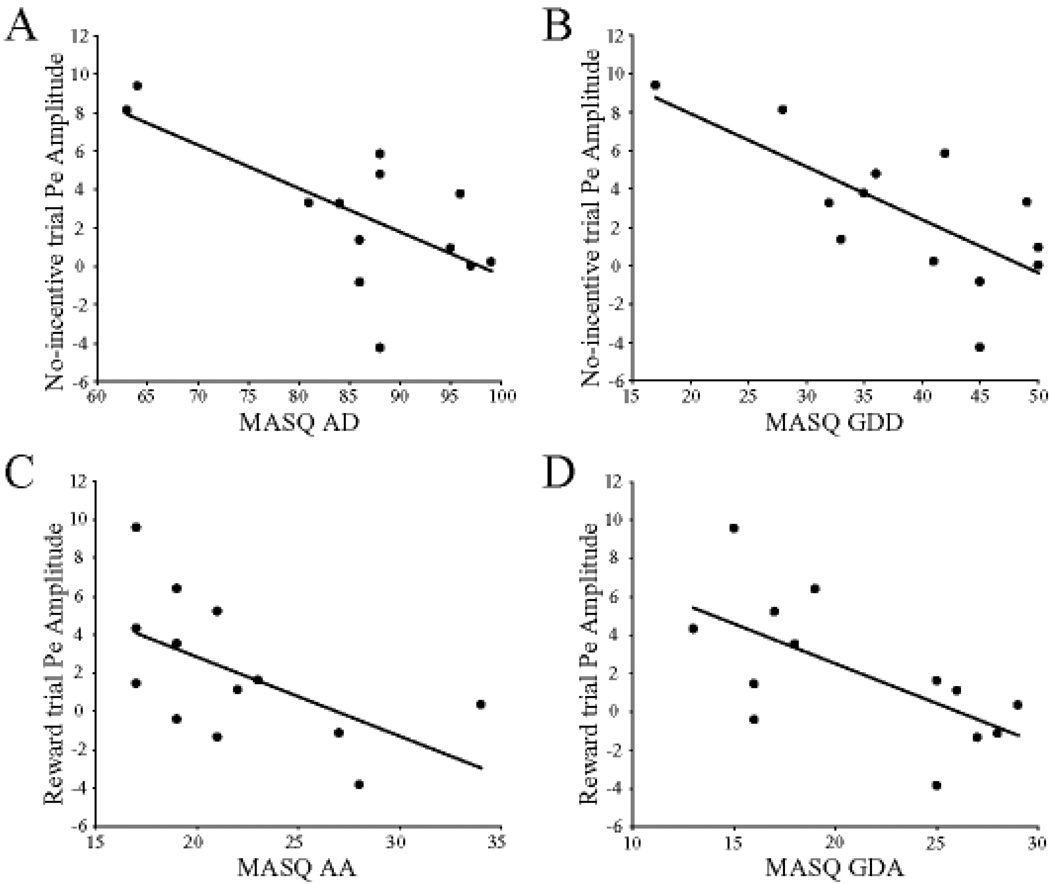
Among MDD subjects, no significant correlations emerged between ERN amplitudes (reward or no-incentive trials) and BDI scores (r < −0.35, ps > 0.23) or any of the MASQ subscales (all rs < −0.45, all ps > 0.12). When considering the Pe, BDI scores were inversely related to Pe amplitudes for both the no-incentive (r = −0.57, p < 0.041; Figure 3A) and reward (r = −0.66, p < 0.015; Figure 3B) trials. For the MASQ subscales, the Pe amplitudes during no-incentive trials were significantly correlated with AD (r = −0.68, p < 0.006; Figure 4A) and GDD (r = −0.72, p < 0.006; Figure 4B) scores, but not with GDA (r = −0.37, p = 0.21) or AA (r = −0.47, p = 0.10) scores. [The correlation involving AD scores was, however, driven by two MDD subjects with low anhedonic symptoms and should thus be interpreted cautiously.] Conversely, for reward trials, Pe amplitudes were significantly correlated with AA (r = −0.58, p < 0.040; Figure 4C) and GDA (r = −0.65, p < 0.020; Figure 4D) scores, but not GDD (r = −0.47, p = 0.10) or AD (r = −0.32, p = 0.27).
Figure 3.
Scatterplots and Pearson correlations between error-positivity (Pe) amplitudes and Beck Depression Inventory (BDI) scores for (A) no-incentive (B) and reward trials. MDD participants only (n = 13).
Figure 4.
Scatterplots and Pearson correlations between error-positivity (Pe) amplitudes and Mood and Anxiety Symptom Questionnaire (MASQ) subscale. (A) anhedonia depression (MASQ AD); (B) general distress depressive symptoms (MASQ GDD); (C) anxious arousal (MASQ AA); (D) general distress-anxious symptoms (MASQ GDA). For (A) and (B), Pe amplitudes were derived from no-incentive trials, whereas for (C) and (D) from reward trials. MDD participants only (n = 13).
Discussion
The overarching goal of the current study was to examine action monitoring, specifically error processing, in unmedicated and moderately depressed participants as a function of task-relevant incentives and clinical profile. Three main findings emerged. First, MDD subjects were characterized by potentiated ERN responses irrespective of task incentives, highlighting generally exaggerated reactivity in early (automatic) stages of error processing. Second, when monetary rewards were available, MDD subjects exhibited reduced Pe amplitudes, highlighting blunted responses at later stages assumed to index conscious error awareness and subjective affective evaluative responses (Falkenstein et al., 2000). Third, whereas ERN amplitudes were not modulated by depression severity or clinical symptoms, significant negative correlations between BDI/MASQ scores and Pe amplitudes emerged, indicating that the most severely depressed subjects were characterized by the lowest Pe responses.
The current ERN findings replicate prior reports of potentiated ERN responses in MDD, particularly in samples tested under no-incentive conditions (Alexopoulos et al., 2007; Holmes & Pizzagalli, 2008; but see Compton et al., 2008; Ruchsow et al., 2004; Ruchsow et al., 2006; Schrijvers et al., 2008; Schrijvers et al., 2009a). Two additional findings extend these prior studies, however. First, ERN differences were not modulated by task-incentives (the significant Group × Response interaction was not qualified by the Group × Response × Condition triple interaction), highlighting global ERN potentiation in moderately depressed and unmedicated MDD subjects. Second, groups did not differ in their CRN amplitudes, suggesting that abnormal action monitoring is specific to error responses at early stages of processing. Of note, a recent study found that the ERN might prime defensive motivational responses (Hajcak & Foti, 2008). Altogether, these findings indicate that depression is characterized by exaggerated automatic responses to perceived failures, which might foster the emergence or maintenance of negative processing biases (Chiu & Deldin, 2007; Holmes & Pizzagalli, 2008). In addition, replicating prior findings (Compton et al., 2008; Holmes & Pizzagalli, 2008; Schrijvers et al., 2008; but see Chiu & Deldin 2007; Schrijvers et al., 2009a), the lack of correlation between ERN amplitude and clinical symptoms is consistent with recent proposals that the ERN may be associated with “stable characteristics related to internalizing disorders” (p. 1349, Olvet and Hajcak, 2008). Reports of increased ERN amplitudes in asymptomatic individuals carrying allelic variations within the promoter region of the serotonin transporter (Althaus et al., 2009; Fallgatter et al., 2004) previously linked to increased vulnerability to depression (Caspi et al., 2003) are further consistent with this argument. Due to the relatively limited sample size investigated here, further studies are needed, however, to further evaluate putative relations between ERN amplitude and clinical profile.
Relative to controls, MDD subjects exhibited significantly lower Pe amplitudes following errors committed in reward trials, while no group differences were observed in the no-incentive condition. Moreover, among the MDD subjects, significant negative correlations between depression severity and Pe amplitudes emerged, consistent with prior reports of reduced Pe in severely depressed (Alexopoulos et al., 2007; Schrijvers et al., 2008). The current finding of decreased Pe in the reward – but not no-incentive – condition is intriguing, particularly when considering recent data in healthy controls indicating that heightened Pe amplitudes occur when errors are motivationally salient (Overbeek et al., 2005) and are associated with increased relevance to task goals (Boksem et al., 2006; Heldmann, Russeler, & Munte, 2005). In MDD, reduced Pe amplitudes are evident in severely depressed samples, a finding hypothesized to stem from increased task disengagement due to apathy/anhedonia (Schrijvers et al., 2008; Schrijvers et al., 2009a). Accordingly, the present data suggest that decreased Pe amplitudes become apparent in moderately depressed individuals when monetary incentives are made available and the relationship between anhedonia and action monitoring is more explicitly probed. The relationships between Pe amplitude and anhedonic symptoms and negative affect among MDD subjects are in line with recent data indicating that the Pe is potentiated in individuals with high reward sensitivity (e.g., Boksem et al., 2006; Boksem et al., 2008) and reduced in those with high negative affect (e.g., Hajcak, McDonald, & Simons, 2004). However, it is important to note that anhedonic symptoms were associated with reduced Pe amplitudes in no-incentive trials (and the correlation was mostly driven by two MDD subjects), whereas increased anxious arousal was unexpectedly linked to reduced Pe amplitudes in reward trials. The reasons for this dissociation are currently unclear, and future research will be necessary to determine how individual differences in self-reported anhedonia and anxiety interact with the action monitoring system.
Generally increased ERN amplitudes and reduced Pe to errors occurring within reward contingencies are consistent with a failure within the MDD group to appropriately react to mistakes in performance and recruit additional resources when motivationally required. Prior research has indicated that MDD subjects are characterized by reduced functional coupling between DLPFC and ACC regions immediately following error commission (Holmes & Pizzagalli, 2008) and the presentation of personally relevant negative stimuli (Siegle et al., 2007). These findings are of interest, particularly when considering the role of ACC and DLPFC regions in cognitive control (Botvinick, Cohen, & Carter, 2004) and reward responsiveness (Kumar et al., 2008). This literature suggests that the present data, and earlier reports of abnormal reactions to errors and negative feedback in depression (Beats et al., 1996; Elliott et al., 1996; Steffens, Wagner, Levy, Horn, & Krishnan, 2001), could emerge as a result of exaggerated paralimbic activation and/or a failure to recruit PFC-based cognitive control, possibly due to abnormal frontocingulate connectivity.
Findings from this study should be interpreted in the context of several limitations. First, while participants exhibited slower RT for incongruent relative to congruent trials and for no-incentive relative to reward trials, no group differences were observed in the behavioral data. While this allows for the ERP data to be interpreted without the possible confounds associated with differences in task performance, it is inconsistent with prior findings of less adaptive incongruence (Stroop) effects in MDD (Holmes and Pizzagalli, 2007). Second, there was an insufficient number of artifact-free ERP segments to perform analyses that concurrently examined Condition (no-incentive, reward), Trial Type (incongruent, congruent), and Response (correct, incorrect), and thus would have allowed us to fully dissociate error processing and conflict monitoring. Finally, clinical symptomatology was assessed exclusively through self-report measures, rather than clinician-rated measures; we cannot exclude that self-reporting biases might partially confound some of the correlations described in the current analyses. Given these limitations, the current findings await replications in larger samples before conclusive interpretations about the possible differential impact of task-relevant incentives on the electrophysiological correlates of action monitoring in MDD can be made.
These limitations notwithstanding, the present findings are consistent with the hypotheses that MDD is associated with a heightened reactivity to error commission and a failure to increase activity in the presence of task-relevant incentives. The inclusion of trial-to-trial reward manipulations uncovered action monitoring dysregulation in the current, unmedicated and moderately depressed sample, with evidence of generally potentiated early (automatic) abnormal reaction to errors (ERN) but blunted responses under reward conditions at later stages associated with motivational significance of errors (Pe). Given that abnormal executive function and ERN/Pe amplitudes have been shown to predict symptom remission and antidepressant treatment response in elderly depressed patients (Alexopoulos et al., 2007; Kalayam & Alexopoulos, 2003; Sneed et al., 2007), additional research examining the relationship between ERP correlates of action monitoring, depression, and task relevant incentives is warranted.
Acknowledgments
This research was supported by a Sackler Scholar in Psychobiology Research Grant (AJH) and NIH grants (1 F31MH078346, AJH; R01MH68376, DAP). The authors wish to thank Ryan Bogdan and Daniel G. Dillon for their contributions and assistance with various aspects of this research. Dr. Pizzagalli has received research support from GlaxoSmithKline and Merck & Co., Inc. and consulting fees from ANT North America Inc. (Advanced Neuro Technology) for studies unrelated to this project.
Footnotes
Mr. Holmes reports no competing interests.
References
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biological Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Kalayam B, Katz R, Kanellopoulos D, et al. Event-related potentials in an emotional go/no-go task and remission of geriatric depression. Neuroreport. 2007;18:217–221. doi: 10.1097/WNR.0b013e328013ceda. [DOI] [PubMed] [Google Scholar]
- Althaus M, Groen Y, Wijers AA, Mulder LJ, Minderaa RB, Kema IP, et al. Differential effects of 5-HTTLPR and DRD2/ANKK1 polymorphisms on electrocortical measures of error and feedback processing in children. Clinical Neurophysiology. 2009;120:93–107. doi: 10.1016/j.clinph.2008.10.012. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, D.C: American Psychiatric Press; 1994. 4 ed. [Google Scholar]
- Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychological Medicine. 1996;26:591–603. doi: 10.1017/s0033291700035662. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Boksem MA, Tops M, Kostermans E, De Cremer D. Sensitivity to punishment and reward omission: evidence from error-related ERP components. Biological Psychology. 2008;79:185–192. doi: 10.1016/j.biopsycho.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Boksem MA, Tops M, Wester AE, Meijman TF, Lorist MM. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Research. 2006;1101:92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain Cognition. 1987;6:175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. American Journal of Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Lin M, Vargas G, Carp J, Fineman SL, Quandt LC. Error detection and posterror behavior in depressed undergraduates. Emotion. 2008;8:58–67. doi: 10.1037/1528-3542.8.1.58. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, McKay AP, Herrod JJ, Robbins TW, Paykel ES. Neuropsychological impairments in unipolar depression: the influence of perceived failure on subsequent performance. Psychological Medicine. 1996;26:975–989. doi: 10.1017/s0033291700035303. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Herrmann MJ, Roemmler J, Ehlis AC, Wagener A, Heidrich A, et al. Allelic Variation of Serotonin Transporter Function Modulates the Brain Electrical Response for Error Processing. Neuropsychopharmacology. 2004;29:1506–1511. doi: 10.1038/sj.npp.1300409. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders -Patient Edition (SCID-I/P, 1/2007 revision) New York: Biometrics Research, New York State Psychiatric Institute; 2007. [Google Scholar]
- Forbes EE, Christopher MJ, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. Journal of Child Psychology and Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganushchak LY, Schiller NO. Motivation and semantic context affect brain error-monitoring activity: an event-related brain potentials study. Neuroimage. 2008;39:395–405. doi: 10.1016/j.neuroimage.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A Neural System for Error-Detection and Compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Foti D. Errors are aversive: defensive motivation and the error-related negativity. Psychological Science. 2008;19:103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, Simons RF. Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology. 2005;42:161–170. doi: 10.1111/j.1469-8986.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40:895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Error-related psychophysiology and negative affect. Brain Cognition. 2004;56:189–197. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Heldmann M, Russeler J, Munte TF. Event-related potentials in a decision-making task with delayed and immediate reward conditions. Journal of Psychophysiology. 2005;19:270–274. [Google Scholar]
- Holmes AJ, Pizzagalli DA. Task feedback effects on conflict monitoring and executive control: relationship to subclinical measures of depression. Emotion. 2007;7:68–76. doi: 10.1037/1528-3542.7.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal Dynamics of Error Processing Dysfunctions in Major Depressive Disorder. Archives of General Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Cohen JD. Errors in reward prediction are reflected in the event-related brain potential. Neuroreport. 2003;14:2481–2484. doi: 10.1097/00001756-200312190-00037. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical Neurophysiology. 2000;111:1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Kalayam B, Alexopoulos GS. A preliminary study of left frontal region error negativity and symptom improvement in geriatric depression. American Journal of Psychiatry. 2003;160:2054–2056. doi: 10.1176/appi.ajp.160.11.2054. [DOI] [PubMed] [Google Scholar]
- Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology. 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- Leuthold H, Sommer W. ERP correlates of error processing in spatial S-R compatibility tasks. Clinical Neurophysiology. 1999;110:342–357. doi: 10.1016/s1388-2457(98)00058-3. [DOI] [PubMed] [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clinical Psychology Review. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek T, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing: On the functional significance of the Pe vis-+á-vis the ERN/Ne. Journal of Psychophysiology. 2005;19:319–329. [Google Scholar]
- Pizzagalli DA, Dillon DG, Bogdan R, Holmes AJ. Reward and punishment processing in the human brain: Clues from affective neuroscience and implications for depression research. In: Vartanian O, Mandel D, editors. Neuroscience of Decision Making. New York: Psychology Press; 2009. [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated subjects with Major Depressive Disorder. American Journal of Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in Major Depressive Disorder: Evidence from a probabilistic reward task. Journal of Psychiatric Research. 2009;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchsow M, Herrnberger B, Beschoner P, Gron G, Spitzer M, Kiefer M. Error processing in major depressive disorder: evidence from event-related potentials. Journal of Psychiatric Research. 2006;40:37–46. doi: 10.1016/j.jpsychires.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Herrnberger B, Wiesend C, Gron G, Spitzer M, Kiefer M. The effect of erroneous responses on response monitoring in patients with major depressive disorder: A study with event-related potentials. Psychophysiology. 2004;41:833–840. doi: 10.1111/j.1469-8986.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- Schrijvers D, de Bruijn ER, Maas Y, De Grave C, Sabbe BG, Hulstijn W. Action monitoring in major depressive disorder with psychomotor retardation. Cortex. 2008;44:569–579. doi: 10.1016/j.cortex.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Schrijvers D, de Bruijn ER, Maas YJ, Vancoillie P, Hulstijn W, Sabbe BG. Action monitoring and depressive symptom reduction in major depressive disorder. International Journal of Psychophysiology. 2009a;71:218–224. doi: 10.1016/j.ijpsycho.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Schrijvers D, Maas YJ, Pier MP, Madani Y, Hulstijn W, Sabbe BG. Psychomotor changes in major depressive disorder during sertraline treatment. Neuropsychobiology. 2009b;59:34–42. doi: 10.1159/000205516. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shalgi S, Barkan I, Deouell LY. On the positive side of error processing: error-awareness positivity revisited. European Journal of Neuroscience. 2009;29:1522–1532. doi: 10.1111/j.1460-9568.2009.06690.x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Sneed JR, Roose SP, Keilp JG, Krishnan KR, Alexopoulos GS, Sackeim HA. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. American Journal of Geriatric Psychiatry. 2007;15:553–563. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Wagner HR, Levy RM, Horn KA, Krishnan KR. Performance feedback deficit in geriatric depression. Biological Psychiatry. 2001;50:358–363. doi: 10.1016/s0006-3223(01)01165-9. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Luu P, Frishkoff G, Quiring J, Poulsen C. Frontolimbic response to negative feedback in clinical depression. Journal of Abnormal Psychology. 2003;112:667–678. doi: 10.1037/0021-843X.112.4.667. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Von Cramon DY. How does error correction differ from error signaling? An event-related potential study. Brain Research. 2006;1105:102–109. doi: 10.1016/j.brainres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]