Abstract
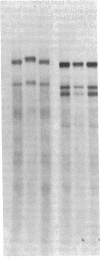
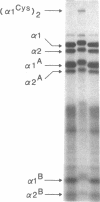
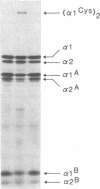
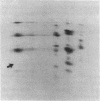
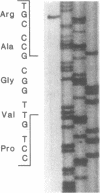
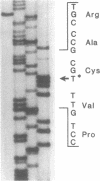
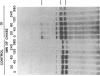
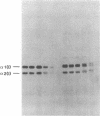
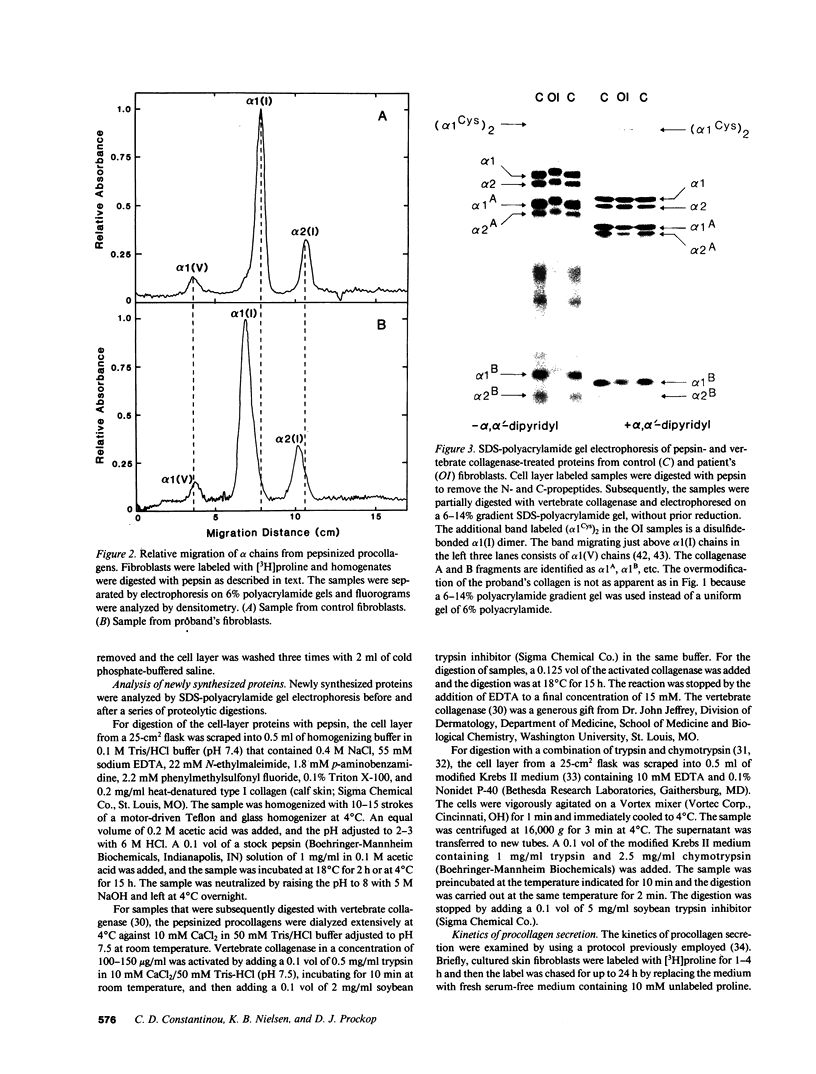
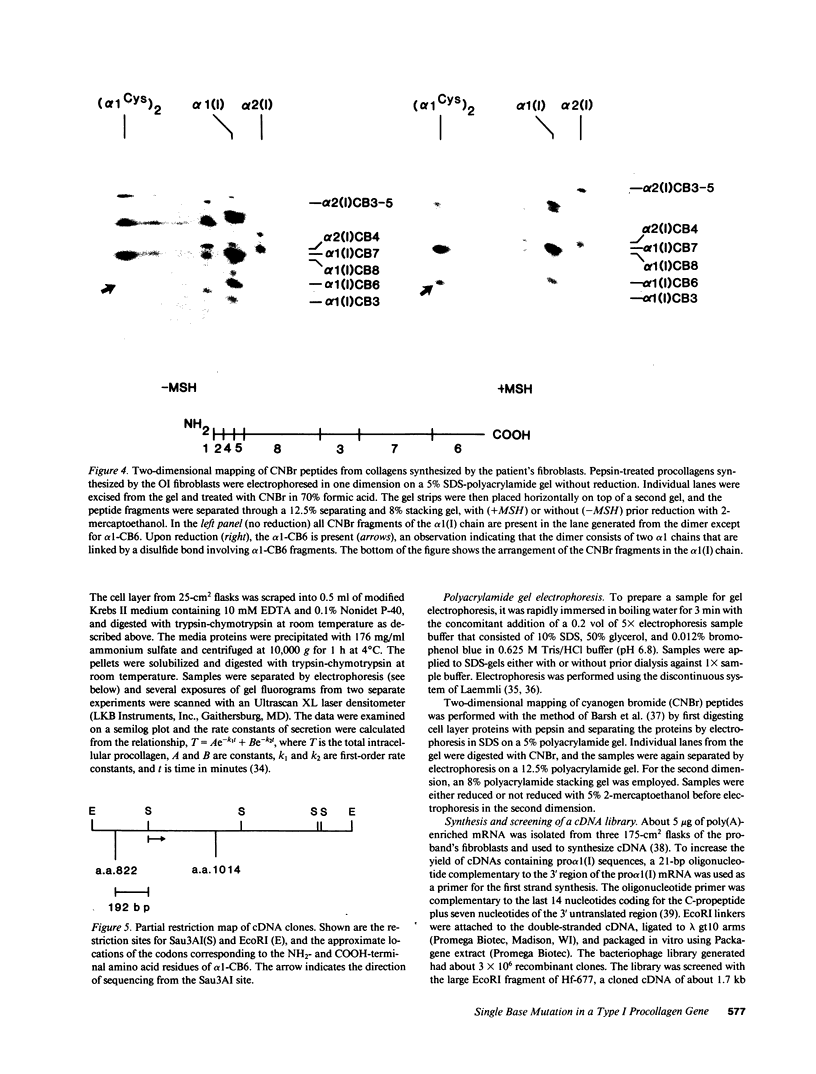
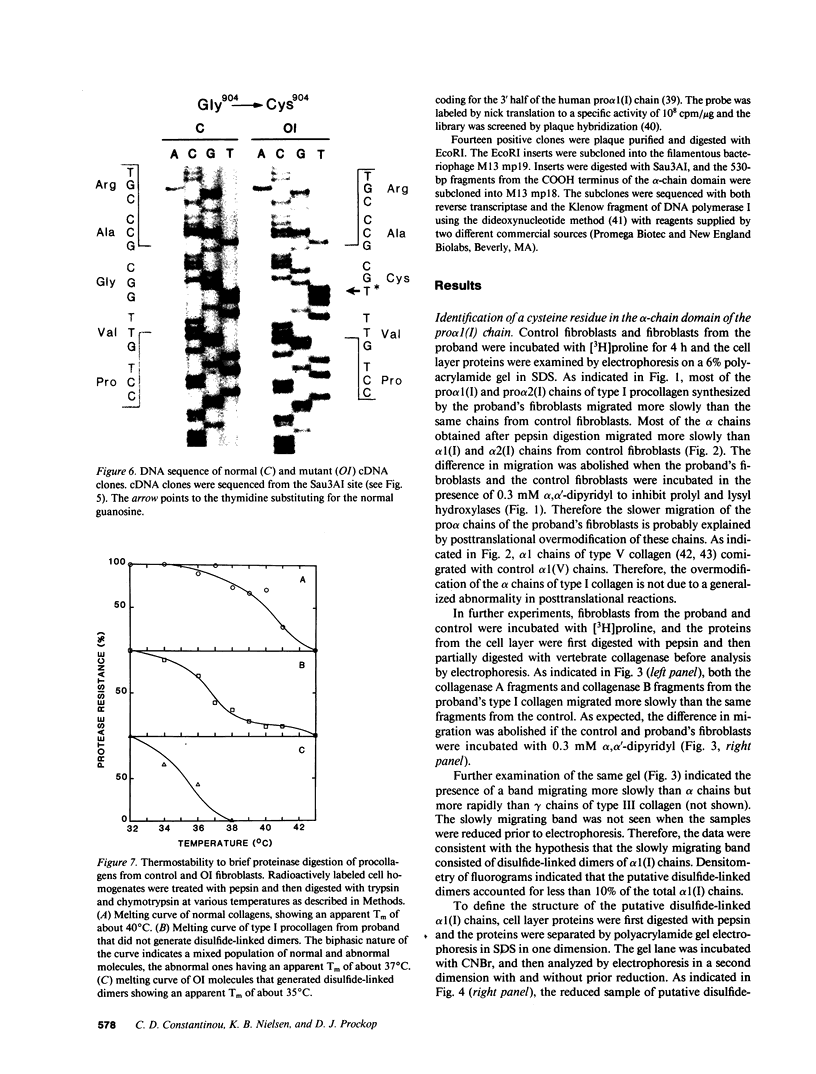
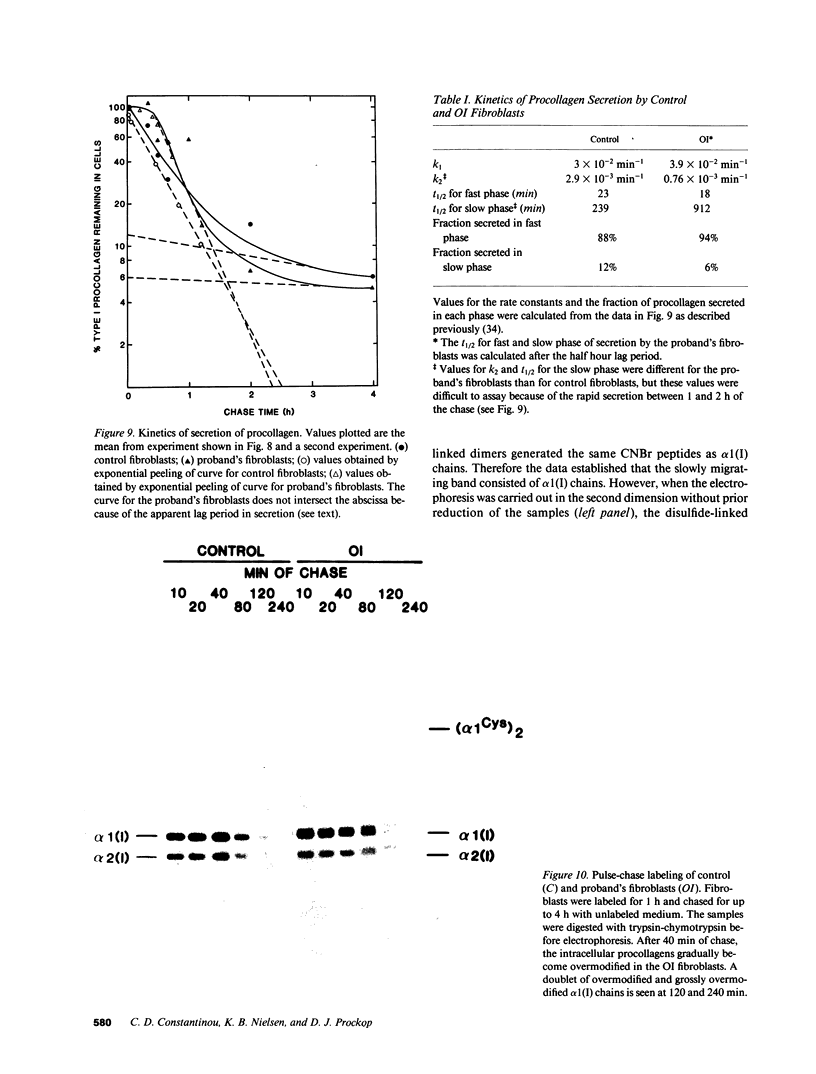
A fraction of the pro alpha 1(I) and pro alpha 2(I) chains in type I procollagen synthesized by the fibroblasts from a proband with a lethal variant of osteogenesis imperfecta were overmodified by posttranslational reactions. After digestion with pepsin, some of the alpha 1(I) chains were recovered as disulfide-linked dimers. Mapping of cyanogen bromide peptides indicated that the disulfide link was contained in alpha 1-CB6, the cyanogen bromide fragment containing amino acid residues 823-1014 of the alpha 1(I) chain. Nucleotide sequencing of cDNA clones demonstrated a substitution of T for G that converted glycine 904 of the alpha 1(I) chain to cysteine. A large fraction of the type I procollagen synthesized by the proband's fibroblasts had a thermostability that was 3-4 degrees C lower than the normal type I procollagen as assayed by brief proteinase digestion. In addition, the type I procollagen synthesized by the proband's fibroblasts was secreted with an abnormal kinetic pattern in that there was a lag period of about 30 min in pulse-chase experiments. The mutation of glycine to cysteine was not found in type I procollagen synthesized by fibroblasts from the proband's parents. Therefore, the mutation was a sporadic one. However, the mother's fibroblasts synthesized a type I procollagen in which part of the pro alpha chains were overmodified and had a lower thermostability. Therefore, the proband may have inherited a mutated allele for type I procollagen from her mother that contributed to the lethal phenotype. The mother was asymptomatic. She was somewhat short and had slightly blue sclerae but no definitive signs of a connective tissue abnormality. The observations on the mother indicated, therefore, that a mutation that causes synthesis of a type I procollagen with a lowered thermal stability does not necessarily produce a heritable disorder of connective tissue.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsh G. S., Byers P. H. Reduced secretion of structurally abnormal type I procollagen in a form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5142–5146. doi: 10.1073/pnas.78.8.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., Peterson K. E., Byers P. H. Peptide mapping of collagen chains using CNBr cleavage of proteins within polyacrylamide gels. Coll Relat Res. 1981 Nov;1(6):543–548. doi: 10.1016/s0174-173x(81)80035-0. [DOI] [PubMed] [Google Scholar]
- Barsh G. S., Roush C. L., Bonadio J., Byers P. H., Gelinas R. E. Intron-mediated recombination may cause a deletion in an alpha 1 type I collagen chain in a lethal form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1985 May;82(9):2870–2874. doi: 10.1073/pnas.82.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Walker I. D., Rogers J. G., Cole W. G. Lethal perinatal osteogenesis imperfecta due to the substitution of arginine for glycine at residue 391 of the alpha 1(I) chain of type I collagen. J Biol Chem. 1987 May 25;262(15):7021–7027. [PubMed] [Google Scholar]
- Bernard M. P., Chu M. L., Myers J. C., Ramirez F., Eikenberry E. F., Prockop D. J. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry. 1983 Oct 25;22(22):5213–5223. doi: 10.1021/bi00291a023. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Eikenberry E. F., Prockop D. J. Formation of the triple helix of type I procollagen in cellulo. A kinetic model based on cis-trans isomerization of peptide bonds. Eur J Biochem. 1981 Sep 1;118(3):607–613. doi: 10.1111/j.1432-1033.1981.tb05562.x. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Prockop D. J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981 Jan 15;110(2):360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Shapiro J. R., Rowe D. W., David K. E., Holbrook K. A. Abnormal alpha 2-chain in type I collagen from a patient with a form of osteogenesis imperfecta. J Clin Invest. 1983 Mar;71(3):689–697. doi: 10.1172/JCI110815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah K. S. Collagen genes and inherited connective tissue disease. Biochem J. 1985 Jul 15;229(2):287–303. doi: 10.1042/bj2290287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Gargiulo V., Williams C. J., Ramirez F. Multiexon deletion in an osteogenesis imperfecta variant with increased type III collagen mRNA. J Biol Chem. 1985 Jan 25;260(2):691–694. [PubMed] [Google Scholar]
- Chu M. L., Williams C. J., Pepe G., Hirsch J. L., Prockop D. J., Ramirez F. Internal deletion in a collagen gene in a perinatal lethal form of osteogenesis imperfecta. Nature. 1983 Jul 7;304(5921):78–80. doi: 10.1038/304078a0. [DOI] [PubMed] [Google Scholar]
- Cohn D. H., Byers P. H., Steinmann B., Gelinas R. E. Lethal osteogenesis imperfecta resulting from a single nucleotide change in one human pro alpha 1(I) collagen allele. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6045–6047. doi: 10.1073/pnas.83.16.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole W. G., Chan D., Chambers G. W., Walker I. D., Bateman J. F. Deletion of 24 amino acids from the pro-alpha 1(I) chain of type I procollagen in a patient with the Ehlers-Danlos syndrome type VII. J Biol Chem. 1986 Apr 25;261(12):5496–5503. [PubMed] [Google Scholar]
- Constantinou C. D., Vogel B. E., Jeffrey J. J., Prockop D. J. The A and B fragments of normal type I procollagen have a similar thermal stability to proteinase digestion but are selectively destabilized by structural mutations. Eur J Biochem. 1987 Mar 2;163(2):247–251. doi: 10.1111/j.1432-1033.1987.tb10794.x. [DOI] [PubMed] [Google Scholar]
- Deak S. B., Nicholls A., Pope F. M., Prockop D. J. The molecular defect in a nonlethal variant of osteogenesis imperfecta. Synthesis of pro-alpha 2(I) chains which are not incorporated into trimers of type I procollagen. J Biol Chem. 1983 Dec 25;258(24):15192–15197. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Kinetics for the secretion of procollagen by freshly isolated tendon cells. J Biol Chem. 1977 Dec 10;252(23):8391–8397. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Dickson L. A., Pope F. M., Korhonen V. R., Nicholls A., Prockop D. J., Myers J. C. Osteogenesis imperfecta: cloning of a pro-alpha 2(I) collagen gene with a frameshift mutation. J Biol Chem. 1984 Nov 10;259(21):12941–12944. [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippola M., Kaffe S., Prockop D. J. A heterozygous defect for structurally altered pro-alpha 2 chain of type I procollagen in a mild variant of osteogenesis imperfecta. The altered structure decreases the thermal stability of procollagen and makes it resistant to procollagen N-proteinase. J Biol Chem. 1984 Nov 25;259(22):14094–14100. [PubMed] [Google Scholar]
- Steinmann B., Nicholls A., Pope F. M. Clinical variability of osteogenesis imperfecta reflecting molecular heterogeneity: cysteine substitutions in the alpha 1(I) collagen chain producing lethal and mild forms. J Biol Chem. 1986 Jul 5;261(19):8958–8964. [PubMed] [Google Scholar]
- Steinmann B., Rao V. H., Vogel A., Bruckner P., Gitzelmann R., Byers P. H. Cysteine in the triple-helical domain of one allelic product of the alpha 1(I) gene of type I collagen produces a lethal form of osteogenesis imperfecta. J Biol Chem. 1984 Sep 10;259(17):11129–11138. [PubMed] [Google Scholar]
- Steinmann B., Tuderman L., Peltonen L., Martin G. R., McKusick V. A., Prockop D. J. Evidence for a structural mutation of procollagen type I in a patient with the Ehlers-Danlos syndrome type VII. J Biol Chem. 1980 Sep 25;255(18):8887–8893. [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Traub W., Steinmann B. Structural study of a mutant type I collagen from a patient with lethal osteogenesis imperfecta containing an intramolecular disulfide bond in the triple-helical domain. FEBS Lett. 1986 Mar 31;198(2):213–216. doi: 10.1016/0014-5793(86)80407-0. [DOI] [PubMed] [Google Scholar]
- Vogel B. E., Minor R. R., Freund M., Prockop D. J. A point mutation in a type I procollagen gene converts glycine 748 of the alpha 1 chain to cysteine and destabilizes the triple helix in a lethal variant of osteogenesis imperfecta. J Biol Chem. 1987 Oct 25;262(30):14737–14744. [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Eisen A. Z. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981 Sep 25;256(18):9511–9515. [PubMed] [Google Scholar]
- Wenstrup R. J., Cohn D. H., Cohen T., Byers P. H. Arginine for glycine substitution in the triple-helical domain of the products of one alpha 2(I) collagen allele (COL1A2) produces the osteogenesis imperfecta type IV phenotype. J Biol Chem. 1988 Jun 5;263(16):7734–7740. [PubMed] [Google Scholar]
- Wenstrup R. J., Tsipouras P., Byers P. H. Osteogenesis imperfecta type IV. Biochemical confirmation of genetic linkage to the pro alpha 2(I) gene of type I collagen. J Clin Invest. 1986 Dec;78(6):1449–1455. doi: 10.1172/JCI112735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. J., Prockop D. J. Synthesis and processing of a type I procollagen containing shortened pro-alpha 1(I) chains by fibroblasts from a patient with osteogenesis imperfecta. J Biol Chem. 1983 May 10;258(9):5915–5921. [PubMed] [Google Scholar]
- Wirtz M. K., Glanville R. W., Steinmann B., Rao V. H., Hollister D. W. Ehlers-Danlos syndrome type VIIB. Deletion of 18 amino acids comprising the N-telopeptide region of a pro-alpha 2(I) chain. J Biol Chem. 1987 Dec 5;262(34):16376–16385. [PubMed] [Google Scholar]
- de Vries W. N., de Wet W. J. The molecular defect in an autosomal dominant form of osteogenesis imperfecta. Synthesis of type I procollagen containing cysteine in the triple-helical domain of pro-alpha 1(I) chains. J Biol Chem. 1986 Jul 5;261(19):9056–9064. [PubMed] [Google Scholar]
- de Wet W. J., Pihlajaniemi T., Myers J., Kelly T. E., Prockop D. J. Synthesis of a shortened pro-alpha 2(I) chain and decreased synthesis of pro-alpha 2(I) chains in a proband with osteogenesis imperfecta. J Biol Chem. 1983 Jun 25;258(12):7721–7728. [PubMed] [Google Scholar]
- de Wett W., Sippola M., Tromp G., Prockop D., Chu M. L., Ramirez F. Use of R-loop mapping for the assessment of human collagen mutations. J Biol Chem. 1986 Mar 15;261(8):3857–3862. [PubMed] [Google Scholar]