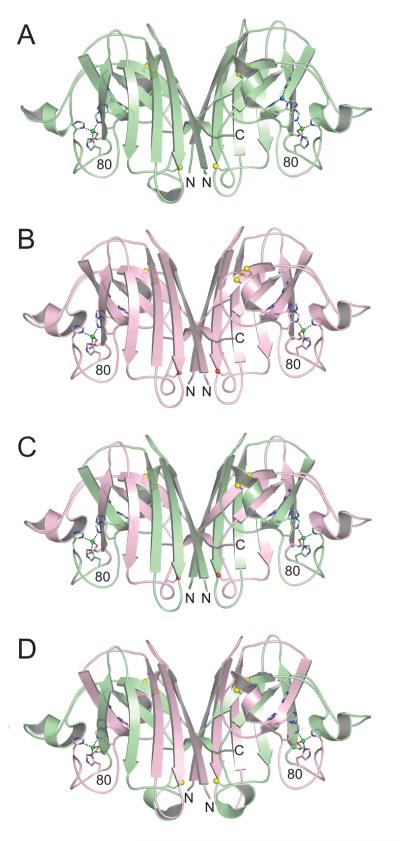
Figure 1.
Structures of human wild type SOD1, mouse wild type SOD1, human (1-80)/mouse (81-153) chimeric SOD1, and mouse (1-80)/human (81-153) chimeric SOD1 in equivalent orientations. A) Human wild type SOD1 homodimer (pdb code 2C9V [30]). The copper and zinc ions are shown as blue and green spheres, respectively. The side chain sulfur atoms of C57, C111, and C146 are shown as yellow spheres. B) Mouse wild type SOD1. The copper-binding sites are devoid of copper, but the zinc-binding sites contain zinc (see text and Figure 2). The side chain oxygen atoms of S111 are shown as red spheres. C) Human (1-80)/mouse (81-153) chimeric SOD1. The color coding is as in panels A) and B), with amino acid residues corresponding to human SOD1 in green and mouse SOD1 in pink. D) Mouse (1-80)/human (81-153) chimeric SOD1. The color coding is as in previous panels.