Figure 6.
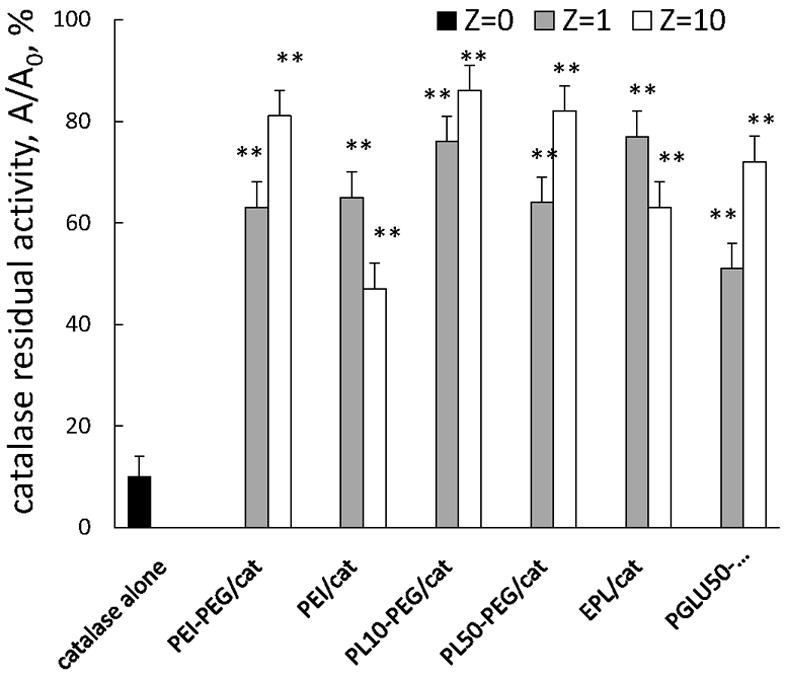
Preservation of catalase enzymatic activity in selected nanozymes. The stability of catalase in BIC was examined upon incubation of different nanozymes (0.5 mg/ml catalase) with trypsin (10-5 M), or pronase (2×10-1 mg/ml) for 3 hours at 37°C. Following incubation, the aliquots were subjected for catalytic activity assessment as described in Figure 4 legend. A residual activity of catalase is expressed as a ratio of enzyme activity after 3h of incubation in the presence of pronase at 37°C to the initial one (at time point 0). Results from N=4 experiments (± SEM) demonstrating that incorporation of catalase into BIC with all studied polymer (with and without PEG) drastically increased stability of catalase against a mixture of proteinases (pronase). Statistical significance is shown by asterisk: P<0.005 (**) compared to “naked” catalase.
