Abstract
BACKGROUND
Androgens and the androgen receptor (AR) play critical roles in the prostate development via mesenchymal-epithelial interactions. Smooth muscle cells, differentiated from mesenchyme, are one of the basic components of the prostate stroma. However, the roles of smooth muscle AR in prostate development are still obscure.
METHODS
We established the smooth muscle selective AR knockout (SM-ARKO) mouse model using the Cre-loxP system, and confirmed the AR knockout efficiency at RNA, DNA and protein levels. Then we observed the prostate morphology changes, and determined the epithelial proliferation, apoptosis, and differentiation. We also knocked down the AR in a prostate smooth muscle cell line (PS-1) to confirm the in vivo findings and to probe the mechanism.
RESULTS
The AR was selectively and efficiently knocked out in the anterior prostates of SM-ARKO mouse. The SM-ARKO prostates have defects with loss of infolding structures, and decrease of epithelial proliferation, but with little change of apoptosis and differentiation. The mechanism studies showed that IGF-1 expression level decreased in the SM-ARKO prostates and AR-knockdown PS-1 cells. The decreased IGF-1 expression might contribute to the defective development of SM-ARKO prostates.
CONCLUSIONS
The AR in smooth muscle cells plays important roles in the prostate development via the regulation of IGF-1 signal.
Keywords: stroma, transgelin, Cre-loxP, gene knockout
INTRODUCTION
The androgen receptor (AR) has been detected in the stromal and epithelial cells of the prostate, and the androgenic effects on prostatic development are mediated via the mesenchymal-epithelial interactions [1–3]. The tissue recombination studies in immune deficient mice showed that urogenital sinus mesenchyme (UGM) could regulate the proliferation and differentiation of urogenital sinus epithelium (UGE) into a well-differentiated secretory epithelium [4–5]. In addition, the recombinants composed of AR negative UGM and wild type (WT) UGE failed to form prostatic structure even in the presence of androgens. These observations initiated people’s attention for the importance of stromal AR. Yet the prostate stroma is heterogeneous, and consists of many cell types, including smooth muscle cells (SMCs), fibroblast cells, endothelial cells, nerve cells, etc. The AR roles in each individual cell type remain to be elucidated.
SMCs, differentiated from the the embryonic mesenchyme, are one of the principle stromal cells in the normal prostate, and are proposed to play roles in the stromal-epithelial interactions, as well as prostate development and cancer [6–9]. The imbalanced interaction between SMCs and epithelium may influence the epithelial function and development [7]. After castration, the prostatic SMCs morphology, transcription, and differentiation have some alterations [10], which suggest the prostatic SMCs’ function could be regulated by the androgen signals. However, the previous studies cannot dissect the AR roles specifically in SMCs or its roles in the epithelium development in mice with intact immune system and physiological prostatic microenvironments.
The Cre-loxP system allows us to selectively knock out the AR in a single cell type, and to study each individual cell type in the prostate. Previously, we have shown that the total AR knockout (ARKO) mice failed to develop prostate [11], but the prostate epithelial ARKO mice developed prostates with loss of differentiation and increase of basal-intermediate cell proliferation [12]. Then we knocked out the fibroblasts AR, and found altered prostate epithelial development with decrease of proliferation, increase of apoptosis, and partial loss of differentiation (manuscript in preparation). To further probe the SMCs AR roles, we established the SMCs conditional AR knockout (SM-ARKO) mouse model, and found the AR loss in SMCs results in abnormal development of the prostate with loss of infolding structures and decrease of proliferation rate, which is mediated by reduced IGF-1. We then knocked down the AR in the smooth muscle cell line PS-1 [13] to confirm the in vivo findings.
MATERIALS AND METHODS
Generation of the SM-ARKO Mice
We mated transgelin-Cre (Tgln-Cre) male mice (C57BL/6*SJL*129S5/SvEvBrd; JAX, Bar Harbor, Maine) with floxed AR (C57BL_6) female mice to generate SMCs-specific AR knockout mice. The generation of Tgln-Cre and floxed AR mice has been described previously [14–16]. Cre and floxed AR alleles in tail genomic DNA of SM-ARKO mice can be detected by polymerase chain reaction (PCR) as described previously [15, 16]. Protocols for use of animals were in accordance with the National Institutes of Health.
Activity of Cre Recombinase in Tissue Sections
The transgenic Tgln-Cre recombinase activity by 6 weeks (wks) of age was confirmed through breeding with the ROSA26-LacZ reporter line. The ROSA26-LacZ reporter line (Jackson Laboratories) harbors a bacterial β-galactosidase (β-Gal) reporter gene, the expression of which requires Cre-mediated deletion of the floxed “stop” sequence separating the ROSA26 promoter and the β-Gal gene [17]. Thus, the β-Gal gene is expressed only where Cre is expressed and active. Fresh dissected tissues from double transgenic mice (Tgln-Cre and ROSA26-LacZ) and ROSA26-LacZ transgenic control mice were frozen in Tissue-Tek, sectioned at 10 µm, and stained by using the β-Gal staining kit from Specialty Media (Billerica) according to the manufacturer’s suggested protocol.
H&E Staining
The tissue sections were dewaxed and rehydrated routinely. The sections were stained in hematoxylin for 5 min, and washed in running tap water for 5 min. Then the sections were stained in eosin for 30 sec, dehydrated, and mounted by routine methods. We then examined and photographed at least 10 fields per each slide. The consistent and representative fields were chosen to present in the figures.
Immunohistochemistry (IHC)
For the prostate tissues, all three prostatic lobes were embedded and sections prepared at 5 µm; for the PS-1 cells, 1×104 cells were seeded on the slides and fixed by 4% paraformaldehyde the next day. Immunostaining was performed as described previously [12]. The antibodies used were anti-AR (C-19, 1:1000, Santa Cruz Biotechnology), anti-Ki67 (1:1000, Novocastra, UK), anti-P63 (1:500, Abcam), and anti-IGF-1 (1:500, Santa Cruz Biotechnology).
Apoptosis Assay
The in situ cell death detection TUNEL kit (Roche Pharmaceuticals) was used for detection of apoptotic cells according to the manufacturer’s instructions. For the positive control, we incubated sections with DNase I (3000 U/ml in 50 mM Tris-HCl/pH 7.5, 100 µg/ml BSA) for 10 min at 15–25 °C to induce DNA strand breaks, prior to labeling procedure. For the negative control, we incubated sections with label solution only (without terminal transferase) instead of TUNEL reaction mixture.
Cell Cultures
The cell line PS-1 was cultured in DMEM (Life Technologies) medium containing 10% fetal bovine serum, and 100 units/ml penicillin/streptomycin. After cloning the AR-shRNA (5′-GTCGGGCCCTATCCCAGTCCCACTTGCTCGAGCAAGTGGGACTGGGATAGGGCTTTTTGAATTCGC-3′) and scrambled RNA into pSuperior-neo vector (Oligoengine), we transfected these two vectors into PS-1 cells separately. The following day, we replaced the standard media with media containing 500 µg/ml G418. After 2 wks selection, all surviving cells were collected for further experiments.
RNA Extraction, Reverse Transcriptase-PCR (RT-PCR), and Quantitative Real-time PCR (Q-PCR)
Total RNA was extracted and purified using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Three µg total RNA was subjected to reverse transcription using Superscript III (Invitrogen). RT-PCR has been described previously [15], using primers from AR exon 1 and exon 3 : AR sense, 5'-TATCCTGGTGGAGTTGTG-3'; antisense, 5'-CAGAGTCATCCCTGCTTC-3' respectively. Q-PCR was performed with first strand cDNA, specific gene primers, and SYBR Green PCR Master Mix (Biorad, Hercules, CA). The Q-PCR was performed on an iCycler iQ Multi-color real-time PCR detection system (Biorad) as described previously [16]. Primer sequences were as follows: Hepatocyte growth factor (HGF): sense, 5′-AGAGGTACGCTACGAAGTC-3′; antisense, 5′-GCTTGCCATCAGGATTGC-3′. Fibroblast growth factor-10 (FGF10): sense, 5′-CTGCTGTTGCTGCTTCTTG-3′; antisense, 5′-TGACCTTGCCGTTCTTCTC-3′. Insulin like growth factor 1 (IGF-1): sense, 5′-GGTGGATGCTCTTCAGTTC-3′; antisense, 5′-TTTGTAGGCTTCAGTGGG-3′. β-actin: sense, 5′-ACCACACCTTCTACAATGAG-3′; antisense, 5′-ACGACCAGAGGCATACAG-3′. All samples were run in triplicate.
Statistics
The data values were presented as the mean ± SD. To compare data between groups, we used a two-sided Student’s t test. *P < 0.05 was considered significant.
RESULTS
Generation of Mice with Selective Knockout of AR in SMCs
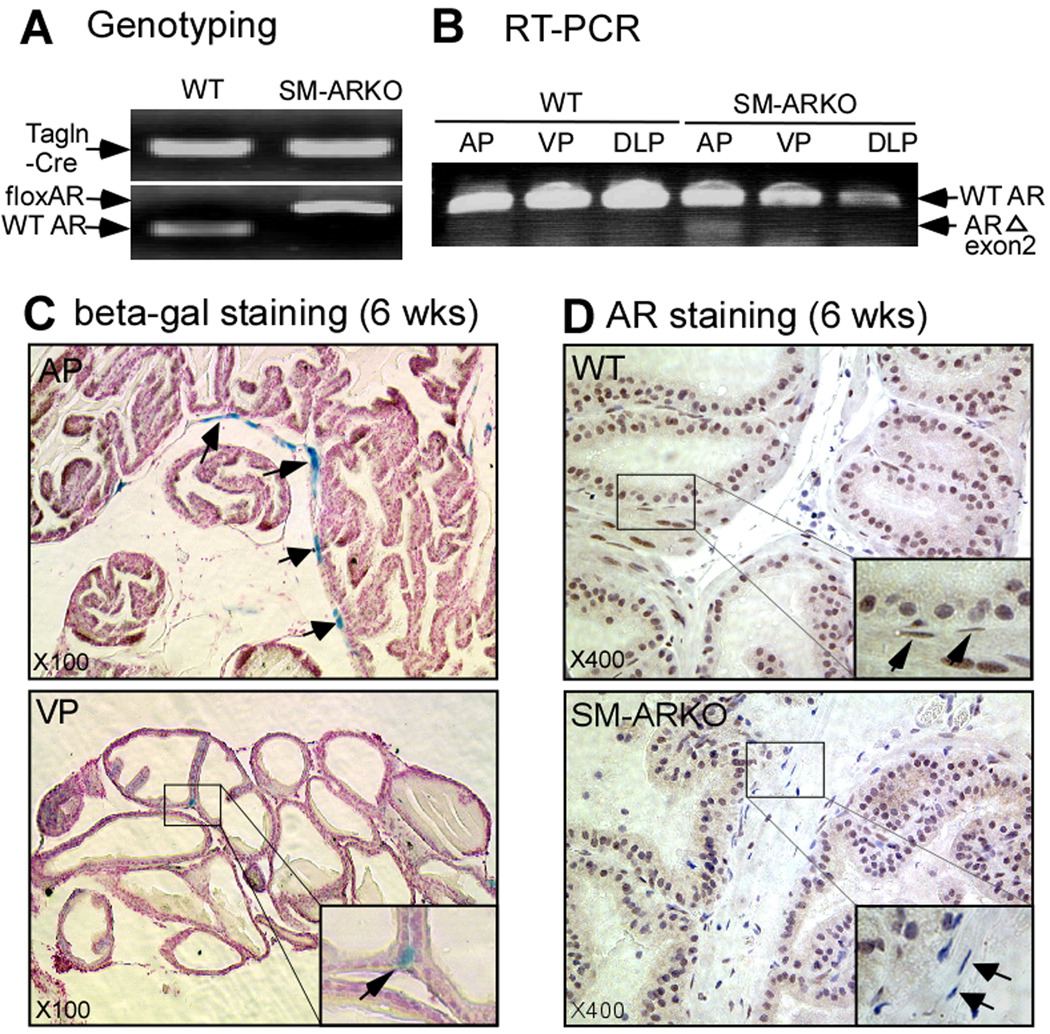
Using a Cre-loxP conditional knockout strategy, we mated female heterozygous flox-AR mice with male Tgln-Cre mice to generate SM-ARKO male mice. The DNA fragments of Cre and floxed AR exon 2 were detected in tail genomic DNA (Fig. 1A).
Fig. 1.
In addition, RNAs from ventral prostate (VP), dorsolateral prostate (DLP), and anterior prostate (AP) were harvested at 12 wks. The AP shows obvious deletion of AR exon 2 with a 220 bp signal from RT-PCR product using primers from exon 1 and exon 3. (Fig. 1B). To further validate the specificity of Cre expression, we crossed the Tgln-Cre mice with the Rosa26R reporter mice [17]. As expected, the prostate epithelial cells have no positive signal, but the SMCs show efficient recombination dominant in the AP, and some in the VP (Fig. 1C). The DLP only has few positive signals (data not shown). Moreover, the IHC staining also showed that the loss of AR was selective in the AP SMCs of SM-ARKO mice, but not in WT mice (Fig. 1D). Taken together, results from genomic DNA genotyping, tissue mRNA RT-PCR assay, Cre reporter assay, and IHC staining all revealed that the AR gene was selectively knocked out in the prostate SMCs.
Morphology Changes of the Prostates of SM-ARKO Mice
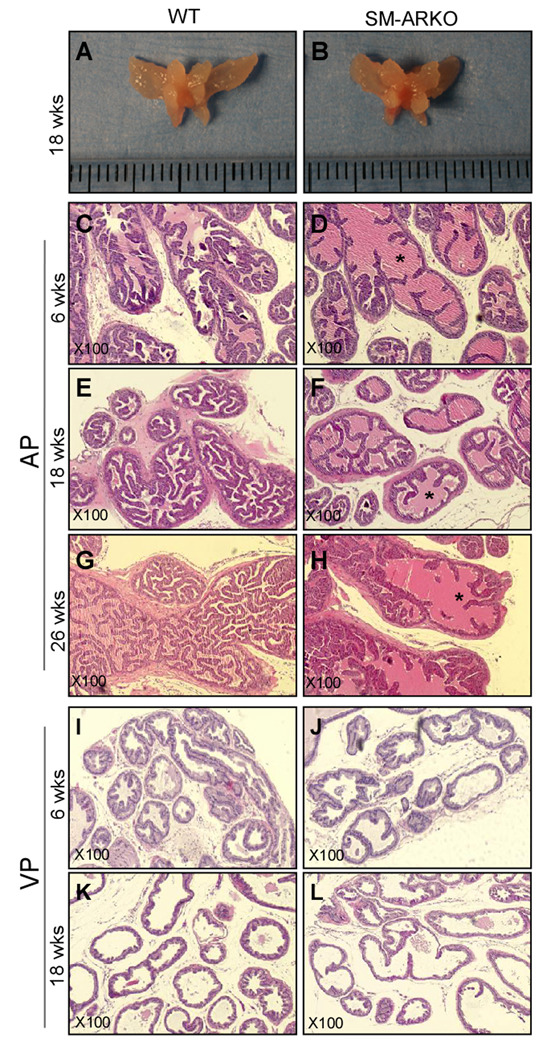
We compared the prostates of SM-ARKO mice and WT littermates, but found no significant difference in gross appearance (Fig. 2A-B) and branching morphogenesis (Fig. S1). However, H&E staining showed defective structures in the SM-ARKO AP with fewer epithelial infoldings into the lumens (Fig. 2C-H). In the VP, we also found fewer epithelial infolding structures and thinner epithelium layer especially in 18-week-old SM-ARKO mice (Fig. 2I-L). In the DLP, we did not find histological changes in SM-ARKO mice (data not shown). The histological changes in different prostate lobes are consistent with the Cre activity: AP > VP >> DLP. Previously, we characterized SM-ARKO mice and their testicular function, and found there is no alteration of the serum testosterone, luteinizing hormone, and follicle stimulating hormone levels [15]. Thus, the morphology changes of SM-ARKO mouse prostates are not due to the hormone level changes.
Fig. 2.
Reduced Epithelial Proliferation in SM-ARKO Prostates
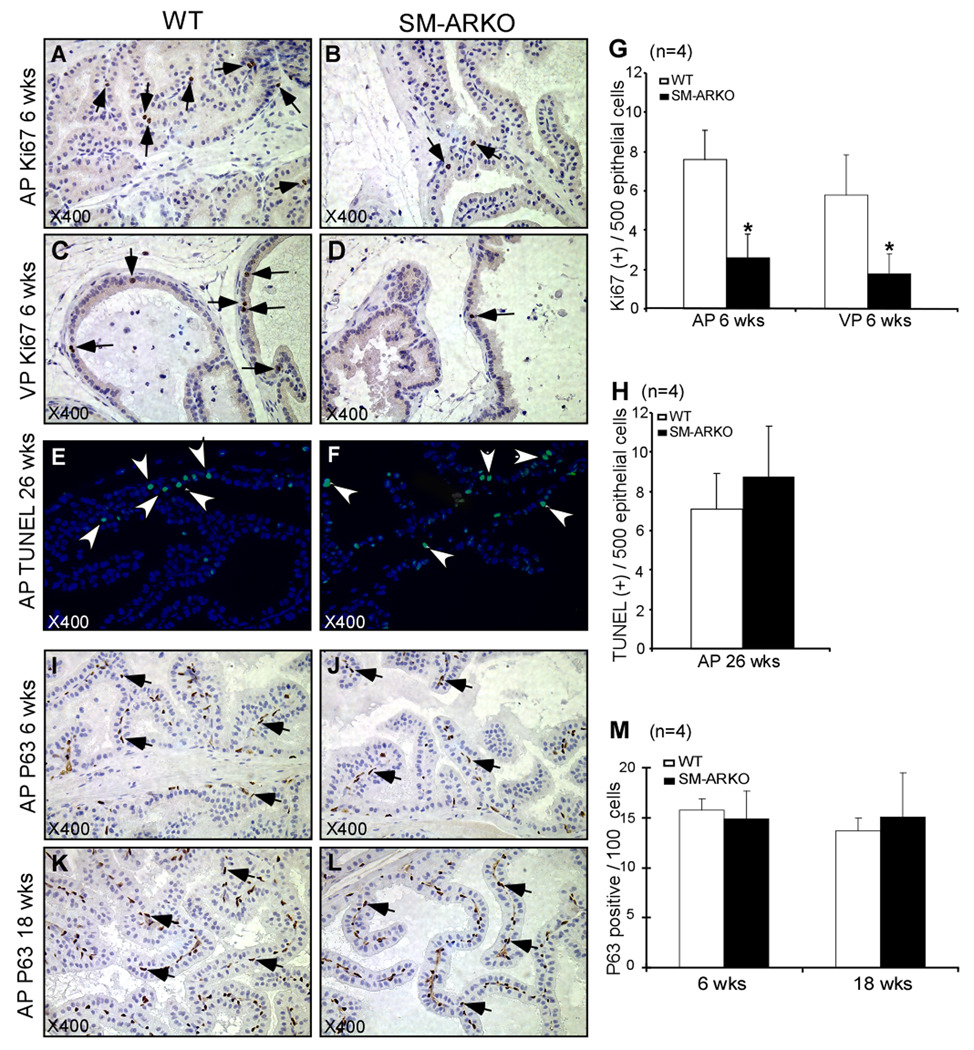
To dissect the mechanism why the SM-ARKO mouse prostates have altered structure with fewer epithelial folding structures, we detected the proliferation, apoptosis, and differentiation of epithelium. Ki67 IHC staining was performed to determine the epithelial proliferation of AP and VP of 6 wks old mice (Fig. 3A-D). Then, we counted 500 epithelial cells to determine the Ki67 positive numbers. In AP lobes, the average Ki67 positive cells were 7.6 ± 1.5 in WT mice, but only 2.6 ± 1.2 in SM-ARKO mice (*P<0.05) (Fig. 3G); in VP lobes, the average of Ki67 positive cells was 5.8 ± 2.03 in WT mice, but 1.8 ± 0.97 in SM-ARKO mice (*P<0.05). The proliferative signals in mouse prostates are high from birth to young adult age (8 weeks). After 12 wks, only few cells are positive for proliferation signals. Therefore, the Ki67 staining at 6 wks old prostates was presented here. In contrast to the proliferation, the apoptotic signal is barely detectable in prostates at a young age. Cellular apoptosis was determine by TUNEL assay, the data did not show significant differences in the AP (Fig. 3E, F, H), VP, or DLP (data not shown) of WT and SM-ARKO mice at 26 wks old. Moreover, we detected the basal cell marker P63 (Fig. 3I-L) and the luminal cell marker keratin 8 (data not shown) using IHC staining. By counting the positive cells per 100 epithelial cells, we did not find any difference of basal cell and luminal epithelial cell ratio between the SM-ARKO and WT mice. Together, the above data indicated that the morphological changes of SM-ARKO mouse prostates are mainly due to the defected epithelium proliferation, not via apoptosis or differentiation.
Fig. 3.
Decreased IGF-1 Expression in SM-ARKO Prostates and AR-knockdown Smooth Muscle Cell Line
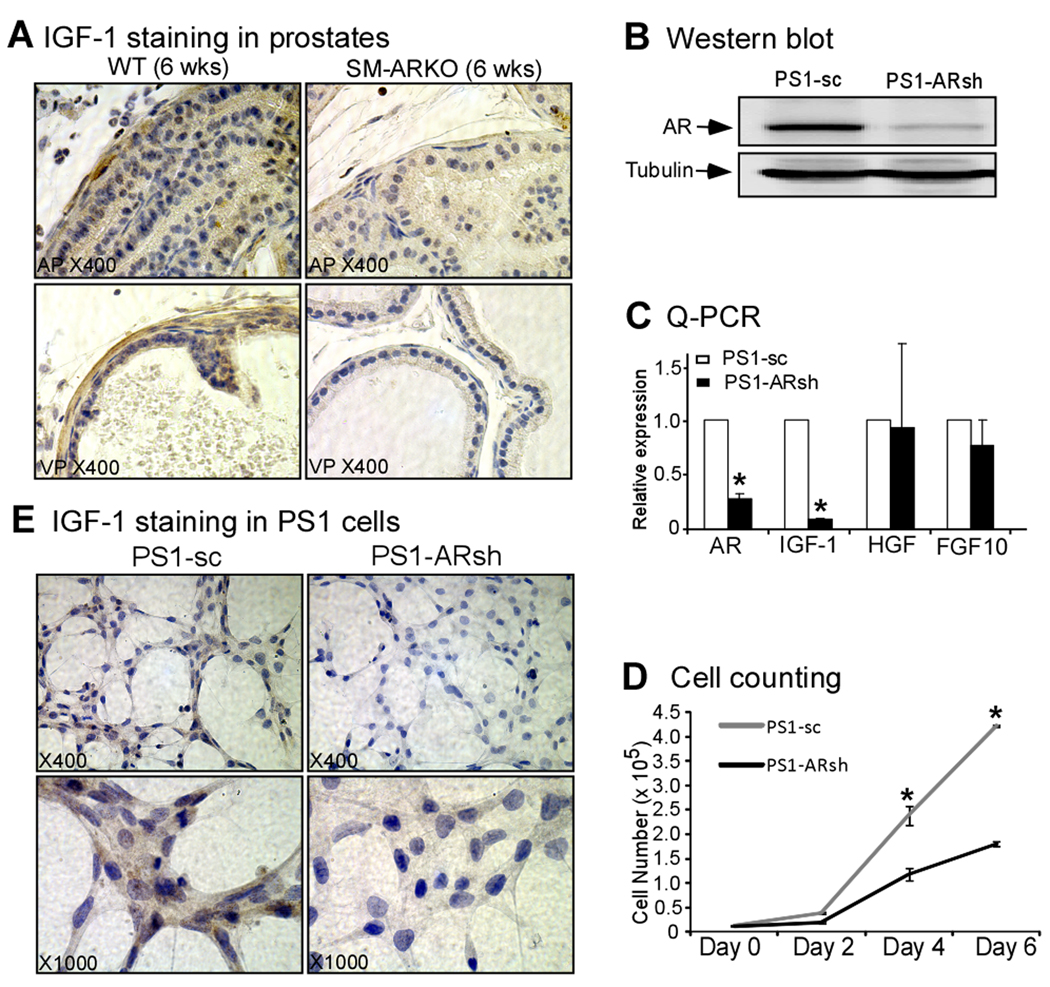
IGF-1 is an important growth factor in prostate for the proliferation and morphogenesis, which was secreted principally in the stromal SMCs [18–20], and is regulated by the AR signal [20–21]. To probe the effect of AR loss in SMCs, we examined the IGF-1 expression levels by IHC staining, and showed a dramatically reduced IGF-1 expression in AP and VP of SM-ARKO mice (Fig. 4A). Then we used an AR positive rat prostate smooth muscle cells line, PS-1 [13], to confirm the in vivo findings. First, we introduced the pSuperior-AR-shRNA/scramble to PS-1 cells, and established the PS-1 AR-shRNA (PS-1-ARsh) and scramble (PS-1-sc) stable cells. Western blotting and Q-PCR were used to confirm the AR knockdown efficiency in PS-1-ARsh cells (Fig. 4B, C). We then compared cellular growth rate between PS-1-sc and PS-1-ARsh cells, and found loss of AR could inhibit the growth of SMCs (Fig. 4D). Furthermore, we used Q-PCR to examine relative gene expression levels of IGF-1, HGF, and FGF10, and found the IGF-1 gene expression level was obviously reduced in PS-1-ARsh cells (Fig. 4C). To confirm the Q-PCR data, we performed the cytohistochemical staining, and also found the lower expression level of IGF-1 in PS-1-ARsh cells (Fig. 4E). These data suggested that SMCs’ AR could regulate the IGF-1 expression level in the prostate, then further regulate the epithelium proliferation.
Fig. 4.
DISCUSSION
The initial observation of prostatic epithelial development via mesenchymal-epithelial interaction was reported two decades ago [3,7,22]. Based on IHC results, AR was detected in urogenital sinus mesenchyme during early development, but not in epithelium [23], indicating the importance of mesenchymal AR in the prostate early development. The tissue recombinants of AR-deficient UGM and WT-epithelium could not form prostate in the nude mice sub-renal capsules, but the recombinants of WT-UGM and AR-deficient epithelium could develop to a prostate-like organ [24–25], which indicated the critical roles of mesenchymal AR in the prostate development. However, the previous tissue recombination assays were conducted in immune-deficient mice without proper prostate microenvironment, and could not determine the AR roles in different cell types of the prostate stroma. In this work, we focused on the SMCs AR role in the prostate development by using the Cre-loxP gene knockout system.
We generated the SM-ARKO mice by mating floxed AR mice [11] with Tgln-Cre mice [14]. The expression pattern in the organs of Tgln-Cre transgenic mice has been reported previously [14,26–27]. In the prostate, the AP has the highest Cre expression level, thus has the highest AR knockout efficiency. Based on the H&E staining, we found that there are reduced infolding structures mostly in AP. Further studies showed the phenotype change is mainly due to the defected epithelium proliferation, not apoptosis. Surprisingly, there is no significant difference in the basal cells ratio between SM-ARKO and WT mice. This suggests that SMCs AR might play more important roles in the epithelium proliferation, rather than in apoptosis or differentiation. However, the AR knockout efficiency, even in AP, is only around 50% (data not shown). The remaining AR positive SMCs might partially maintain the basic AR function, thus the prostates could still develop and grow with normal gross appearance.
The molecular pathways of prostate stromal AR regulating epithelial development are mediated, at least in part, by stromal growth factors under the influence of androgen signals [28]. The IGF-1, primarily mediated through the IGF-1 receptor 1 (IGF-R1), is important for the prostate normal development and cancer [29–30], and is synthesized principally by the SMCs [19–20]. Androgen/AR signal could regulate IGF-1 expression directly at the transcriptional level via potential androgen response elements located on the IGF-1 5′ promoter [21]. In this study, we found the IGF-1 expression level (based on IHC staining data) was obviously reduced in the SM-ARKO prostates, which might contribute to the altered prostate infolding structure and reduced epithelial proliferation. To further confirm the in vivo findings, we cultured the prostate smooth muscle PS-1 cells, and found knockdown of AR could also decrease the IFG-1 expression level, but not the FGF10 or HGF.
We determined the IGF-1, FGF7, FGF10, HGF, and smooth muscle alpha-actin (SMA) expression levels in 26-week-old mouse APs using Q-PCR, but only found a significant difference of SMA expression (Fig. S2, n=5). The decreased SMA expression in SM-ARKO AP could be due to the growth inhibition of SMCs when knocking out the AR. The in vitro PS-1-ARsh/sc growth data (Fig. 4D) further support this result. There are two possible explanations for why we did not observe the changes of those growth factors: (1) SMCs are not the only cell source to produce those growth factors in prostate, and (2) the SMC population in the whole prostate is no more than 10%, and the presence of many other cell types could mask the result. Therefore, the Q-PCR data collected from the whole AP did not show the difference.
Taken together, by using this SM-ARKO mouse model, we found the AR in SMCs plays important roles in the epithelial cells normal development. The morphological changes are mainly due to the reduced epithelium proliferation mediated by IGF-1 signal.
Supplementary Material
Acknowledgments
This work was partly supported by NIH Grants CA127300 and CA137474, George H. Whipple Professorship Endowment, and Taiwan Department of Health Clinical Trial and Research Center of Excellence Grant DOH99-TD-B-111-004.
Footnotes
Disclosure statement: None of the authors have anything to declare.
REFERENCES
- 1.Thomson AA. Mesenchymal mechanisms in prostate organogenesis. Differentiation. 2008;76(6):587–598. doi: 10.1111/j.1432-0436.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- 2.Cunha GR, Alarid ET, Turner T, Donjacour AA, Boutin EL, Foster BA. Normal and abnormal development of the male urogenital tract. Role of androgens, mesenchymal-epithelial interactions, and growth factors. J Androl. 1992;13(6):465–475. [PubMed] [Google Scholar]
- 3.Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee HJ, Wang C, Mizokami A. Androgen receptor: an overview. Crit Rev Eukaryot Gene Expr. 1995;5(2):97–125. doi: 10.1615/critreveukargeneexpr.v5.i2.10. [DOI] [PubMed] [Google Scholar]
- 4.Chung LW, Cunha GR. Stromal-epithelial interactions: II. Regulation of prostatic growth by embryonic urogenital sinus mesenchyme. Prostate. 1983;4(5):503–511. doi: 10.1002/pros.2990040509. [DOI] [PubMed] [Google Scholar]
- 5.Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation. 2008;76(6):578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro E, Becich MJ, Hartanto V, Lepor H. The relative proportion of stromal and epithelial hyperplasia is related to the development of symptomatic benign prostate hyperplasia. J Urol. 1992;147(5):1293–1297. doi: 10.1016/s0022-5347(17)37546-8. [DOI] [PubMed] [Google Scholar]
- 7.Cunha GR, Hayward SW, Dahiya R, Foster BA. Smooth muscle-epithelial interactions in normal and neoplastic prostatic development. Acta Anat (Basel) 1996;155(1):63–72. doi: 10.1159/000147791. [DOI] [PubMed] [Google Scholar]
- 8.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76(6):641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166(6):2472–2483. [PubMed] [Google Scholar]
- 10.Antonioli E, Della-Colleta HH, Carvalho HF. Smooth muscle cell behavior in the ventral prostate of castrated rats. J Androl. 2004;25(1):50–56. doi: 10.1002/j.1939-4640.2004.tb02758.x. [DOI] [PubMed] [Google Scholar]
- 11.Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99(21):13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu CT, Altuwaijri S, Ricke WA, Huang SP, Yeh S, Zhang C, Niu Y, Tsai MY, Chang C. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci U S A. 2007;104(31):12679–12684. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerdes MJ, Dang TD, Lu B, Larsen M, McBride L, Rowley DR. Androgen-regulated proliferation and gene transcription in a prostate smooth muscle cell line (PS-1) Endocrinology. 1996;137(3):864–872. doi: 10.1210/endo.137.3.8603596. [DOI] [PubMed] [Google Scholar]
- 14.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A. 2002;99(10):7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, Yeh S, Chen YT, Wu CC, Chuang KH, Lin HY, Wang RS, Chang YJ, Mendis-Handagama C, Hu L, Lardy H, Chang C. Oligozoospermia with normal fertility in male mice lacking the androgen receptor in testis peritubular myoid cells. Proc Natl Acad Sci U S A. 2006;103(47):17718–17723. doi: 10.1073/pnas.0608556103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeh S, Hu YC, Wang PH, Xie C, Xu Q, Tsai MY, Dong Z, Wang RS, Lee TH, Chang C. Abnormal mammary gland development and growth retardation in female mice and MCF7 breast cancer cells lacking androgen receptor. J Exp Med. 2003;198(12):1899–1908. doi: 10.1084/jem.20031233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 18.Kleinberg DL, Ruan W, Yee D, Kovacs KT, Vidal S. Insulin-like growth factor (IGF)-I controls prostate fibromuscular development: IGF-I inhibition prevents both fibromuscular and glandular development in eugonadal mice. Endocrinology. 2007;148(3):1080–1088. doi: 10.1210/en.2006-1272. [DOI] [PubMed] [Google Scholar]
- 19.Ohlson N, Bergh A, Stattin P, Wikstrom P. Castration-induced epithelial cell death in human prostate tissue is related to locally reduced IGF-1 levels. Prostate. 2007;67(1):32–40. doi: 10.1002/pros.20480. [DOI] [PubMed] [Google Scholar]
- 20.Ohlson N, Bergh A, Persson ML, Wikstrom P. Castration rapidly decreases local insulin-like growth factor-1 levels and inhibits its effects in the ventral prostate in mice. Prostate. 2006;66(16):1687–1697. doi: 10.1002/pros.20368. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Zhao W, Zhao J, Pan J, Wu Q, Zhang Y, Bauman WA, Cardozo CP. Identification of androgen response elements in the insulin-like growth factor I upstream promoter. Endocrinology. 2007;148(6):2984–2993. doi: 10.1210/en.2006-1653. [DOI] [PubMed] [Google Scholar]
- 22.Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7(3):248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- 23.Cooke PS, Young P, Cunha GR. Androgen receptor expression in developing male reproductive organs. Endocrinology. 1991;128(6):2867–2873. doi: 10.1210/endo-128-6-2867. [DOI] [PubMed] [Google Scholar]
- 24.Cunha GR, Chung LW. Stromal-epithelial interactions--I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981;14(12):1317–1324. doi: 10.1016/0022-4731(81)90338-1. [DOI] [PubMed] [Google Scholar]
- 25.Cunha GR, Donjacour A. Stromal-epithelial interactions in normal and abnormal prostatic development. Prog Clin Biol Res. 1987;239:251–272. [PubMed] [Google Scholar]
- 26.Frutkin AD, Shi H, Otsuka G, Leveen P, Karlsson S, Dichek DA. A critical developmental role for tgfbr2 in myogenic cell lineages is revealed in mice expressing SM22-Cre, not SMMHC-Cre. J Mol Cell Cardiol. 2006;41(4):724–731. doi: 10.1016/j.yjmcc.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 27.Umans L, Cox L, Tjwa M, Bito V, Vermeire L, Laperre K, Sipido K, Moons L, Huylebroeck D, Zwijsen A. Inactivation of Smad5 in endothelial cells and smooth muscle cells demonstrates that Smad5 is required for cardiac homeostasis. Am J Pathol. 2007;170(5):1460–1472. doi: 10.2353/ajpath.2007.060839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry PA, Maitland NJ, Collins AT. Androgen receptor signalling in prostate: effects of stromal factors on normal and cancer stem cells. Mol Cell Endocrinol. 2008;288(1–2):30–37. doi: 10.1016/j.mce.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Ruan W, Powell-Braxton L, Kopchick JJ, Kleinberg DL. Evidence that insulin-like growth factor I and growth hormone are required for prostate gland development. Endocrinology. 1999;140(5):1984–1989. doi: 10.1210/endo.140.5.6721. [DOI] [PubMed] [Google Scholar]
- 30.Furukawa J, Wraight CJ, Freier SM, Peralta E, Atley LM, Monia BP, Gleave ME, Cox ME. Antisense oligonucleotide targeting of insulin-like growth factor-1 receptor (IGF-1R) in prostate cancer. Prostate. 2010;70(2):206–218. doi: 10.1002/pros.21054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.