Abstract
Changes in fetal magnetocardiographic (fMCG) signals are indicators for fetal body movement. We propose a novel approach to reliably extract fetal body movements based on the field strength of the fMCG signal independent of its frequency. After attenuating the maternal MCG, we use a Hilbert transform approach to identify the R-wave. At each R-wave, we compute the center-of-gravity (cog) of the coordinate positions of MCG sensors, each weighted by the magnitude of the R-wave amplitude recorded at the corresponding sensor. We then define actogram as the distance between the cog computed at each R-wave and the average of the cog from all the R-waves in a 3-min duration. By applying a linear de-trending approach to the actogram we identify the fetal body movement and compare this with the synchronous occurrence of the acceleration in the fetal heart rate. Finally, we apply this approach to the fMCG recorded simultaneously with ultrasound from a single subject and show its improved performance over the QRS-amplitude based approach in the visually verified movements. This technique could be applied to transform the detection of fetal body movement into an objective measure of fetal health and enhance the predictive value of prevalent clinical testing for fetal wellbeing.
Keywords: Center-of-gravity, Hilbert transform, Fetal magenetocardiogram, Fetal body movement, Ultrasound
INTRODUCTION
Fetal movement detection is currently based on maternal perception or Doppler ultrasound. However, the ability of the women to perceive movement varies widely among individuals.14 The normal number of movements and the normal duration of movement have not been defined. Because of the lack of a consistent objective quantification of fetal movement, it has not been well correlated with prenatal outcome. Truly objective assessment of fetal movement is dependent on ultrasound technology, which generally limits the observer to visual assessment of a single parameter, either heart rate or movement, at a time. The bulk of the heart rate monitoring equipment, usually placed on the maternal abdomen directly over the fetal thorax, limits free movement of the ultrasound probe and prohibits optimal viewing, such that simultaneous use of this equipment is not preferable or practical for clinical use. However, there are custom made ultrasounds available to simultaneously collect the fetal heart rate (fHR) and movement.17 Two studies have investigated fetal actocardiogram, which combines ultrasound measurement of fetal movement (the actogram) and fHR acceleration (the cardiogram), which can better predict the neonatal outcome than standard clinical methods.2,17
Over the past two decades, fetal magnetocardiogram (fMCG) has shown to be a viable technique to study fetal heart dynamics.3,13,16,24,28,31 Several methods utilizing multi-dimensional fMCG data have been proposed to extract and study the fHR.4,10,32,33 One of the key features of the fHR is the expression of sleep–wake cycles. Using ultrasound studies, Nijhuis has classified fHR into four different patterns, A, B, C, and D.21 Pattern A is characterized by a stable heart rate with occasional accelerations/decelerations with small oscillation bandwidth of less than 5 beats per minute (bpm). Pattern C is characterized by a stable heart rate with no accelerations with oscillation bandwidth slightly greater than 5 bpm. Pattern B is characterized by a varying heart rate with frequent acceleration/decelerations with wider oscillation bandwidth greater than 5 bpm. Pattern D is characterized by an unstable heart rate with frequent long lasting and large accelerations from the baseline with a wider oscillation bandwidth of greater 10 bpm. By combining the simultaneous occurrence of body movements, the patterns B and D are classified, as 2F (active sleep) and 4F (active awake) behavioral states, respectively. The pattern A along with incidental body movements is termed as 1F (quiet sleep) and the pattern C with no movements is termed as 3F (quiet awake). The traditional fHR analyses performed on the whole heart rate tracings are now applied to the classified fHR patterns to better understand the fetal maturation.15,25 Thus, a reliable identification of fetal movement is essential to study the fetal behavioral states.
Changes in the fMCG are indicators of fetal body movement. These changes could be due to either the movement-induced physiological change of the heart activity or changes in the amplitude of the recorded heart activity caused by the dislocation of the fetal body or the combination of both. Owing to the high time and spatial resolution of fMCG, this technique has been used to construct the actocardiogram in two studies.11,33 The variation of the amplitude of the R-wave with time from a single sensor is used to define actogram in the original approach33 and we denote this approach as actogramR. Instead of using a single R-wave, the changes in the morphology of the QRS complex are used to construct the actogram in the latter version.11 That is, for each sensor, the maximum and minimum values of all the QRS complexes are identified. To quantify the fetal body movement, moving standard deviations are computed with a window covering 20 beats for all the maxima and the minima, independently and summed together. The three sensors with the largest amplitude on the summed standard deviations are averaged to define the actogram. Thus, in this approach more than one sensor is used to construct the actogram and we denote this as the actogramQRS approach.
Both approaches work on the amplitude of the fMCG signal, which is a relative quantity that depends on the position and orientation of the fetus to the pickup coil. In actogramR, one can clearly distinguish between the fetal body movement and non-movement segments when both occur in the data. However, one of the limitations of this approach is that during segments without movements, which can occur in the normal healthy fetus due to the physiologic sleep states, it is not possible to resolve a baseline reference value. In the actogramQRS approach, only the three sensors with largest amplitude are used and hence only the dominant fetal body movements will be detected and weak movements reflected in other sensors may not be captured. To resolve these limitations, we propose a novel approach to compute the actogram by combining both the magnetic field strength of the fMCG signal (independent of its frequency) and its spatial location from all the sensors. To this end, we use a linear de-trending approach to distinguish the instances of fetal body movement from non-movement periods. For the rest of the article, we only take actogramQRS into consideration as it is the modified version of actogramR and compare it with our proposed approach.
MATERIALS AND METHODS
Subjects and Data Collection
Using a 151 SQUID (Superconducting QUantum Interference Device) array system, we collected 39 fMCG data between 30 and 37 weeks of gestation from 27 pregnant women who delivered healthy singleton neonates at term. This study was approved by the local Institutional Review Board and all subjects gave their informed written consent to participate in the study. Each study lasted approximately 20–30 min depending on maternal comfort and the data was recorded with a sampling rate of 312.5 Hz. The data was bandpass filtered between 1 and 50 Hz using the Butterworth filter with zero-phase distortion and the interfering maternal cardiac signal was attenuated using the signal space projection technique.30 The fetal R-waves were calculated using the Hilbert transform approach32 and was followed by an adaptive scheme to correct for the missed and extra beats.27
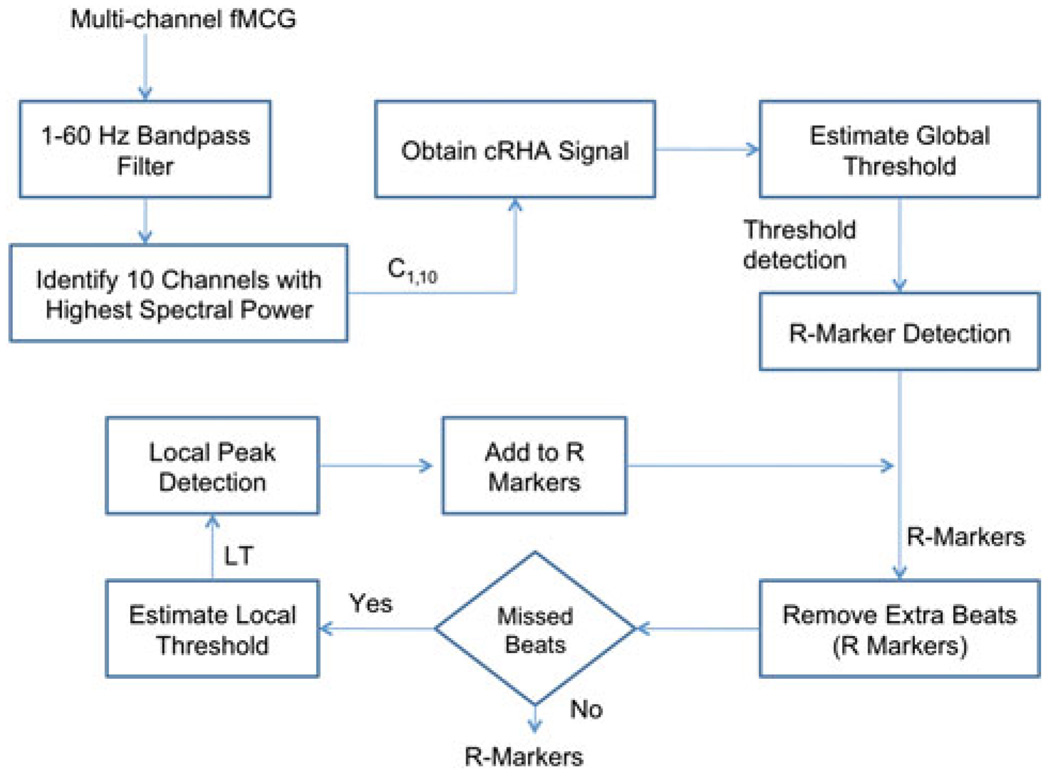
The different steps involved in the identifying the R-wave are shown in a flowchart (see Fig. 1). After attenuation of the maternal cardiac signals using the orthogonal projection technique, we computed the Fourier transform of the resulting signals from all the sensors and selected only the 10 sensors (C1,10) with the highest Fourier peak power in the 1–60 Hz band. Then, for the data x(t) from a sensor we computed Hilbert transform h(t) using the “Hilbert” function in Matlab (Mathwork, Inc.). We define R(n), the rate of change of Hilbert amplitude (RHA) as follows:
R(n) is positive definite and using this property, we linearly combined the RHA from all the 10 sensors with highest spectral content (cRHA).
FIGURE 1.
Flowchart to identify the R-markers in fMCG. C1,10 indicate the 10 channels with highest power in 1–60 Hz frequency band. cRHA is the sum of the RHA of the first 10 ten sensor selected in step 3 of the algorithm.
Estimate the Global Threshold
To this end, we identify the “R” wave of the cardiogram using a threshold detection algorithm. We varied the threshold, identified the “R” wave and used the threshold that yielded the minimum number of missed and extra beats. The extra beat (beats more than 200 bpm) was captured because of the choice of small threshold and missed beat (beats less than 80 bpm) was captured by the choice of the large threshold.
Missed Beat Detection
We identified a local threshold value by computing the average of the previous 10 R-wave amplitudes from the current missed beat location. The missed beats were detected by adaptively lowering the local threshold value for this time region.
Extra Beat Elimination
In this process, if any extra beats were identified because of the use of low threshold, we removed them by comparing this R instance with the instances of R-waves on either side of it. If these time differences fall outside the range of 80–200 beats, then the current beat is discarded.
By denoting τj as the time of occurrence of the jth R-wave, we compute the heart rate (in bpm) at this instance as follows: 60/(τj − τj−1), where the unit of τ is in seconds.
Methodology
For an R-wave at jth time, we compute the center-of-gravity (cog) as follows:
where is the amplitude of the R-wave in the ith sensor, (xi, yi, zi) are the coordinates of this sensor and N represents the number of sensors. Note that cogj is a three dimensional vector. We compute this quantity for every R-wave in the data. We define the actogram as the distance between the cog at each R-wave time point and the mean value of the cog in the current 3-min window. The choice of 3-min window is based on the stability time frame defined by Nijhuis to reliably quantify a fetal behavioral state.21
Indentifying Accelerations in the Heart Rate and Movement in the Actogram
The step-by-step procedure used to construct the actogram is as follows:
Both the heart rate and the actogram are smoothed with a median filter of window length of 32 data points (approximately 12.8 s) in the forward and the reverse direction to avoid phase distortion.
Using τj as the actual sampling time, the heart rate and the actogram are interpolated using cubic-spline function to convert them into continuously sampled data with a sample rate of 312.5 Hz.
A floating baseline for both heart rate and actogram is computed as a linear fit to the data for a window of 2 min with 15 s overlap. As the accelerations in the heart rate and in the actogram vary between 1 and 2 min, this choice of window duration will be appropriate to avoid over fitting of the data and simultaneously capture the inherent fluctuations.
The data is then de-trended using the floating baseline and the segments that are greater than zero are investigated for possible acceleratory phases in the heart rate and substantial movements in actogram.
We calculate the average of the boundaries of the segment and subtract it from the segment so as to enhance the inherent fluctuations in the data. To this end, a root mean square (RMS) value is computed to quantify the magnitude of the inherent variation in the data.
In the heart rate data at each identified location, the RMS value above a certain threshold (4.4 bpm) along with a variation of 10 bpm of the original (unsmoothed) heart rate23 from the floating baseline is used as a decision criterion to detect the inherent accelerations present in it.
Similarly, in the actogram at each identified location, a RMS value above a certain threshold (0.12 cm) is used as a decision criterion to detect the substantial movement in the actogram. This choice of threshold is selected to make the approach highly sensitive and capture the small movements.
We will denote the actogram constructed using this approach by actogramCOG. In the next section, we demonstrate the application of the novel approach to track fetal movement and mark the movements using this automated scheme. We would like to note that only the actogramCOG is smoothed in the above steps and the actogramQRS is calculated based on the original description.11
RESULTS
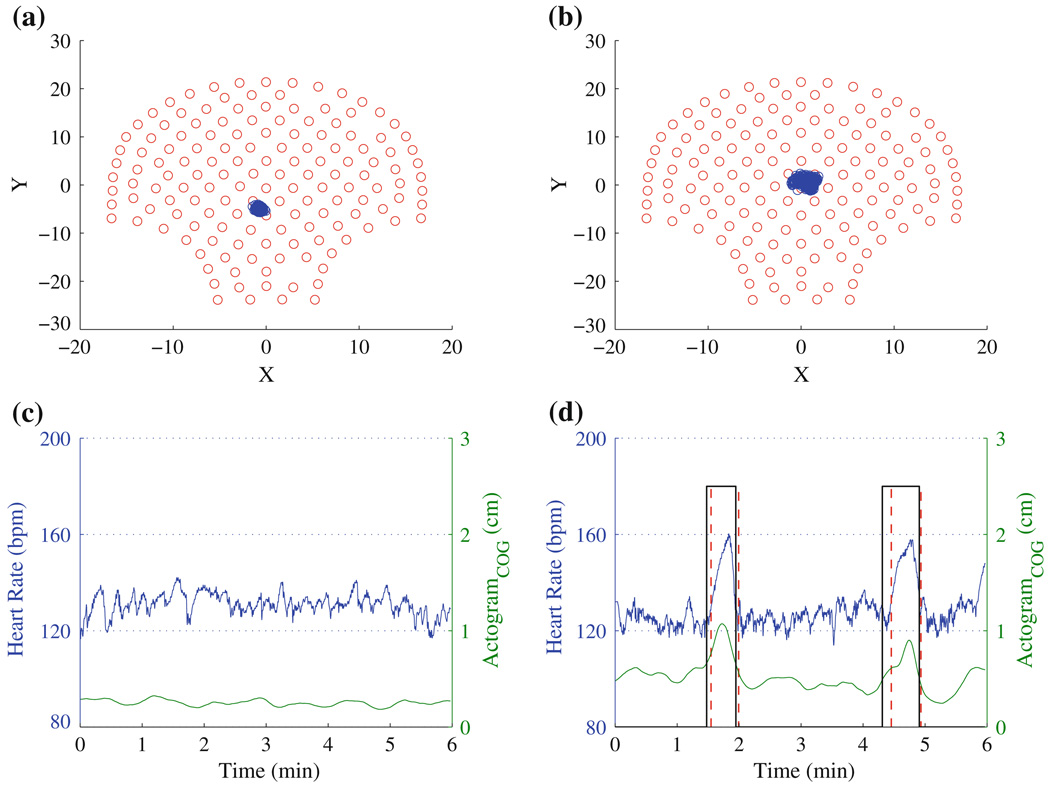
In Fig. 2, we demonstrate the methodology of the actogramCOG using 6 min of data from two fetuses. The cog computed for a fetus displaying a stable heart rate and for a fetus displaying accelerations in heart rate are shown in Fig. 2. The distribution of cog in the sensor space is narrower for the fetus with the stable heart rate compared to the fetus with accelerations in heart rate. Thus, using this approach it is possible to understand the spatial relocation and the extent of the displacement of the fetus from the reference (average) point. In Fig. 2c, there is no acceleration in the fHR nor is there fetal movement, while in Fig. 2d there are two accelerations in the fHR with simultaneous fetal movement. A key feature of the current approach is that we could express the fetal movement in the normalized scale of 0–3 cm, which can help to distinguish the instances of movements from non-movement periods. As the actogramCOG is defined as the weighted average of the absolute amplitude of the “R” wave and the coordinate position of sensors, it will reliably detect the fetal movement if the sensors cover the abdominal area containing fetal heart signals. Thus, this approach can be applied to fMCG from other systems with a different number of sensors (< 150) to reliably quantify the movement.
FIGURE 2.
The distribution of cog (on the 151-sensor array) obtained from two different fetuses: (a) while at rest and (b) during movement. The corresponding actocardiograms are shown in (c) and (d), respectively. The vertical solid and dashed lines represent significant acceleration detected in fHR and the significant movement detected using the proposed approach, respectively.
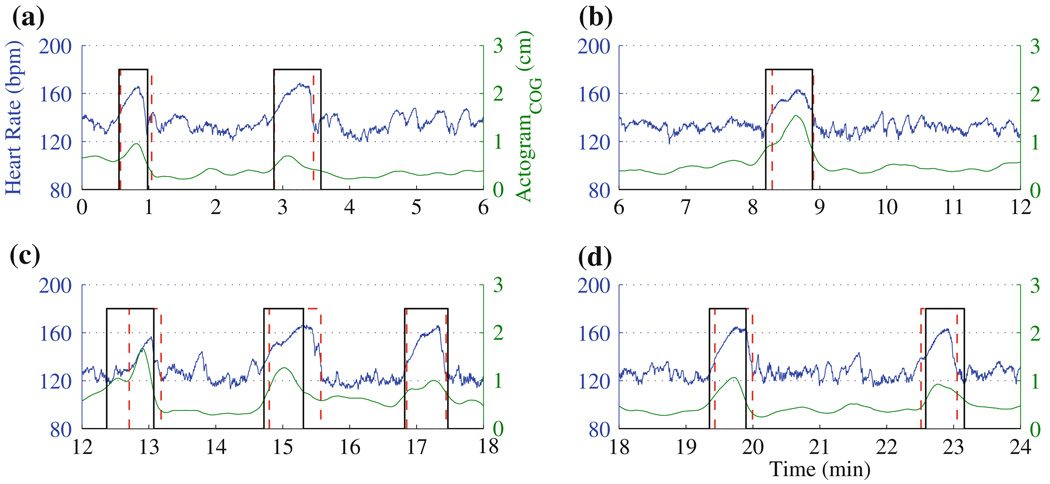
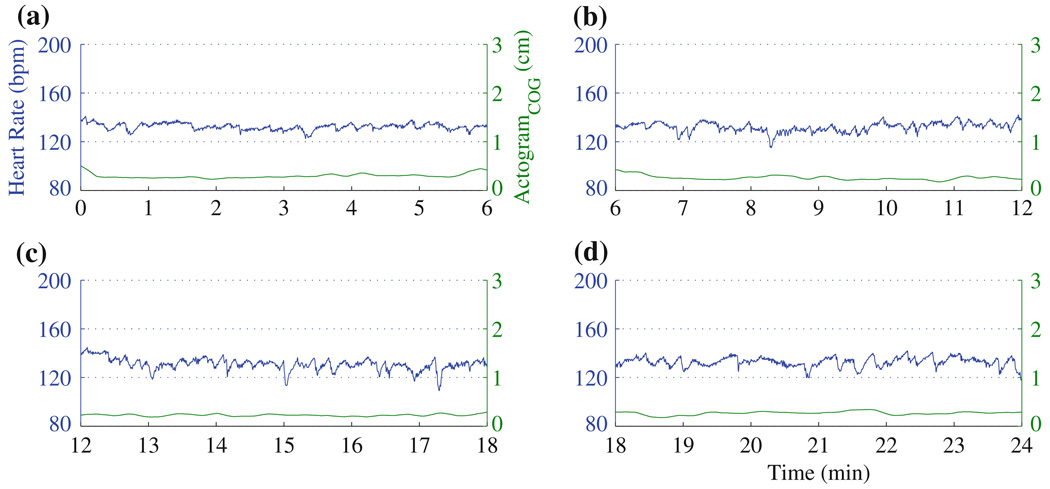
In Figs. 3 and 4, we discuss examples from two fetuses studied for a period of 24 min.
FIGURE 3.
Actocardiogram computed from a 24 min duration fMCG signal from a fetus at 37 weeks of gestational age. In (a)–(d), the results are presented in 6-min windows. For the definition of the vertical lines, see Fig. 2d.
FIGURE 4.
Actocardiogram computed from a 24 min fMCG of a fetus in 33 weeks of gestational age. As shown in Fig. 2, for the sake of clarity the data was partitioned into 6-min windows and shown in (a)–(d). The heart rate is stable throughout the study and there is no significant movement associated with it.
In Fig. 3, we present the actocardiogram computed for a 24-min fMCG signal from a fetus at 37 weeks of gestational age. For the sake of clarity, the data was partitioned into disjoint windows of 6-min duration. This fetus exhibits accelerations in heart rate with simultaneous movement. In Figs. 3a–3d, there is a very good temporal correlation between the accelerations in the heart rate and the movements in the actograms.
In Figs. 4a–4d, the heart rate is stable. The actogram shows no significant deviation from the baseline indicating absence of movement, as one would expect to accompany the stable heart rate. As there is no appreciable deviation from the baseline activity, the algorithm did not mark any movement.
To assess the performance of the actogramCOG, we performed simultaneous ultrasound recordings with fMCG signals from a single subject. Ultrasound video is saved in MPEG format with 30 frames per second and 640 × 480 pixel resolution. Each frame of the ultrasound video recordings is processed using Sticks filter with a template size of 17 and thickness of one to reduce speckle noise in the ultrasound images.5 A horizontal only Sobel operator with a filter size of 11 is applied to the ultrasound images to enhance the fetal thorax edges.12 The fetal movement signal is obtained by following the frame-by-frame displacement of the fetal thorax. Typically, fetal movement signal is contaminated with periodic maternal and fetal breathing artifacts. This signal is then filtered using fourth order Butterworth 0.15 Hz low pass filter to eliminate movements other than fetal body movements.
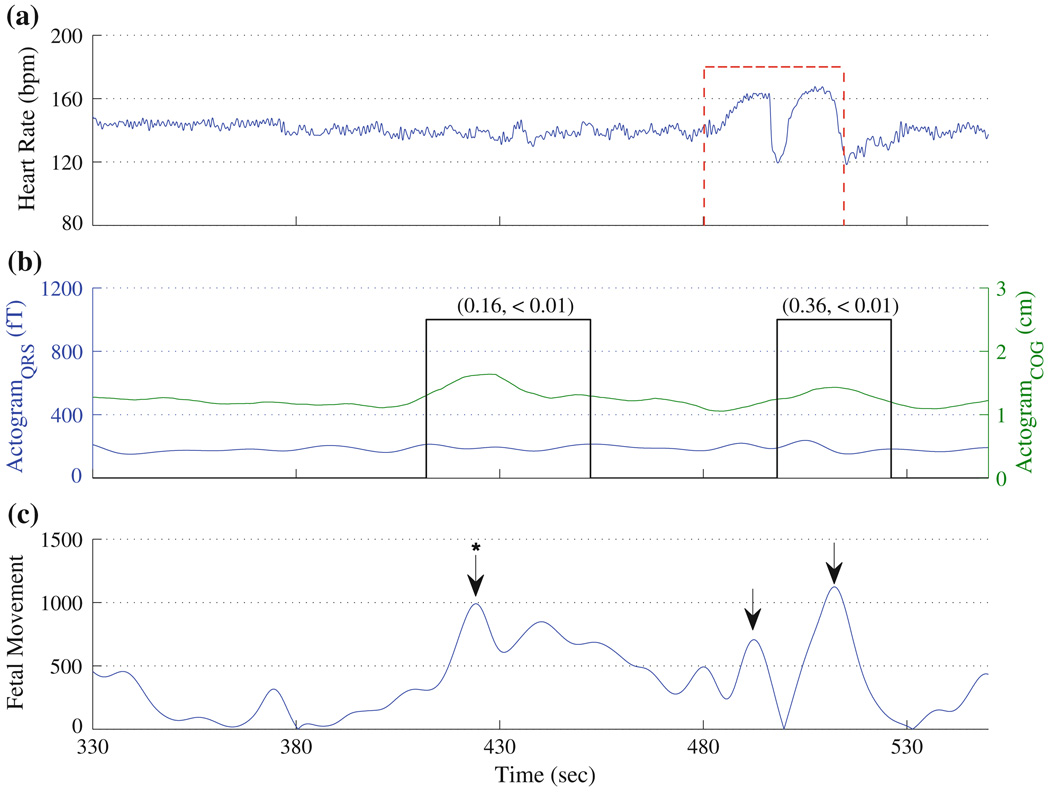
In Fig. 5, we compare the actogramCOG with the actogramQRS. Figure 5a shows the heart rate of the fetus and Fig. 5b shows the actogramCOG along with actogramQRS. Figure 5c shows the fetal movement extracted from the ultrasound images. This fetus displayed a stable heart rate between 330 and 450 s and a large acceleration between 480 and 530 s. Based on the heart rate, one would assume that the fetus has moved only at the later time period. However, this fetus exhibits movements in three instances, which are indicated with arrows. In Fig. 5c, the movement at 424 s (arrow with asterisk) indicates the fetal movement caused by the maternal movement. The actogramCOG clearly shows a significant deviation from the baseline activity to all the movements characterized using ultrasound. However, actogramQRS has captured only the movement exhibited between 480 and 530 s and clearly missed the movement at 424 s. Further, the amplitude of the movement detected using actogramQRS is minimal, and hence difficult to perceive. Thus, the actogramCOG is highly sensitive to the weak movements while actogramQRS is not.
FIGURE 5.
Comparison of the actograms obtained using the actogramCOG and the actogramQRS: (a) Heart rate, (b) actogram, and (c) fetal movement quantified using ultrasound. The arrows indicate the time points noted as fetal movements by a clinical expert blinded to the results of the study. The “*” (at 424 s) indicates the fetal movement caused by maternal movement. In (a), the significant acceleration in fHR is shown by vertical dashed line. In (b), the significant movement detected using actogramCOG, is indicated by solid vertical lines. In (b), the y-axis range for the actogramQRS is adapted from the original work.11 The correlation coefficient and p value between the actogramCOG and actogramQRS in the instances of the fetal movements measured using ultrasound (shown in arrows) and also obtained objectively using actogramCOG are given in the inset.
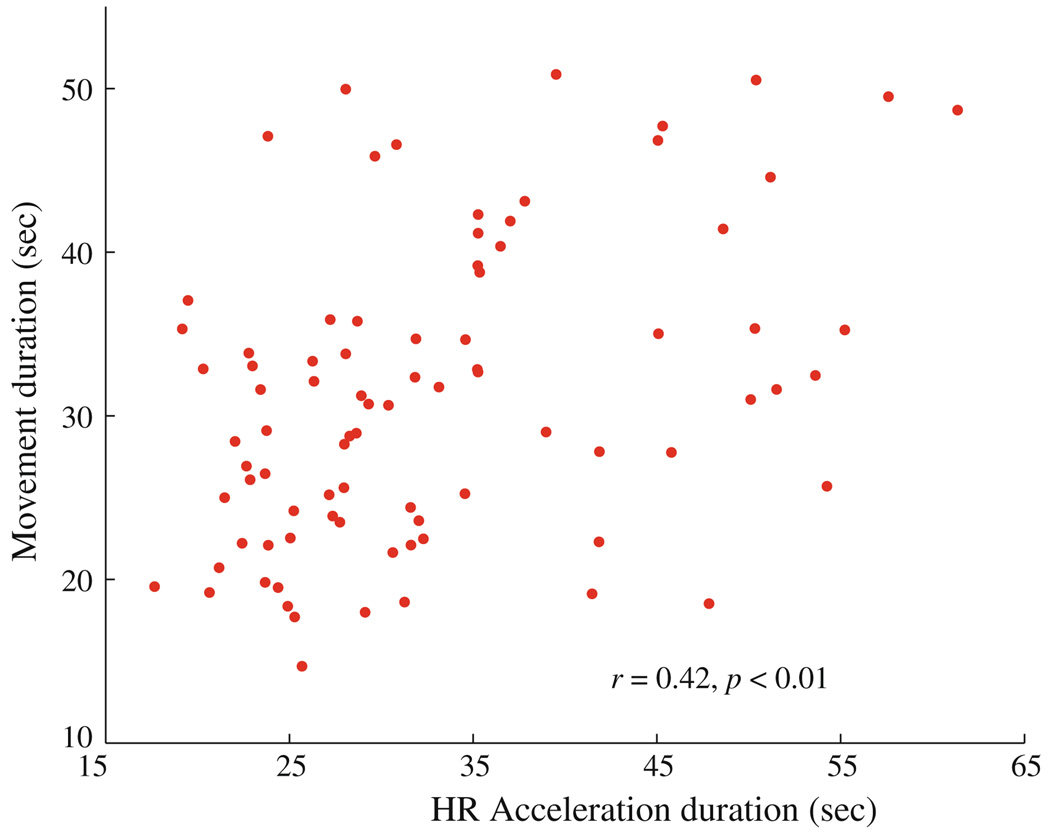
In order to study the degree of coupling between heart rate and movement, we investigated the correlation between the duration of the fetal movement and the duration of the parallelized heart rate acceleration (Fig. 6). There is a statistically significant positive correlation between these two durations (r = 0.42; p < 0.01).29 In addition, we computed the percentage of occurrence of movement in each data and studied its correlation with gestational age. We found that the number of movements decreased with gestational age; however, this trend is not statistically significant.
FIGURE 6.
The correlation between the duration of the fetal movement quantified using the actocardiogram and the duration of the co-occurring heart rate accelerations. The correlation coefficient (r) and the associated probability (p) are given in the inset.
DISCUSSION
Movement is a quintessential behavior of the human fetus. It has been postulated that movements of the embryo and fetus are a fundamental expression of early neural activity.7 The onset of general movements of the head, trunk, and extremities occurs at 7.5–8.5 weeks in the low-risk fetuses delivering at term as healthy infant6 and continues until delivery.22 A maternal perception of decreased fetal movement often precedes fetal death20; therefore, general instruction on “kick counts” is given at routine obstetric visits. Women at greater than 28 weeks gestation who perceive less than 10 movements in 2 h are generally instructed to seek immediate care.1
The current sonographic technique for assessment of fetal health is well-described as part of the “biophysical profile,” which utilizes a single observer watching for evidence of fetal movement and fetal tone over a 30-min period. Specifically, one or more episodes of fetal extremity or spine extension with return to flexion is considered adequate evidence of fetal tone, and three or more discrete body or limb movements within 30 min of observation is considered adequate evidence of fetal movement. These observations are combined with information about fetal breathing and amniotic fluid volume (also obtained ultrasonographically during the 30 min biophysical profile), and fHR recording (obtained separately) to give a risk estimation of intrauterine fetal hypoxia. The advantage of using fetal magnetocardiography with the actogramCOG method is that we can objectively quantify fetal movement without the bias of an observer, and correlate it with the heart rate.
The actogramCOG proposed here will be used in future applications to study the coupling between fetal movement and fHR that occurs in the normal fetus as a part of neural development. Alterations of this coupling have been observed with pregnancy complications, 9 but have not been well-described due to lack of reliable methodology. The actogramCOG method outlined here could be used to determine time-dependent cross-correlation coefficients between fHR and fetal body movement, with the results hopefully yielding prognostic information about pregnancy outcome and likelihood of fetal asphyxia. Ultimately, if validated, these materials and methods could be adapted for clinical use to aid the clinician in determining when delivery of the compromised fetus is indicated. Further, we do not anticipate the fetal magnetoencephalogram (MEG) system to monitor just one aspect of fetal health since this will not be cost-effective. The fetal MEG system can be envisioned as a maternal-fetal physiograph which can record all aspects of fetal well being including the fetal brain, heart, movement, breathing and uterine contractions and their interactions that can aid in better monitoring especially in the case of high-risk pregnancies.
As maternal perception of fetal movement is variable and difficult to objectively quantify, studies have not clearly concluded that there is any utility to the common practice of movement counting.18 However, the actogram, as used here, relies on the objective and quantifiable measurement of fetal movement. In addition, with simultaneously computed reliable fHR, the fMCG analysis lends further insight into the fetal behavioral state at the time of testing.
Comparison of actogramCOG to actogramQRS showed that the former is able to capture almost all the movements in the data in a reliable fashion while the later misses certain movements. ActogramQRS is limited due to fact that it considers only the three sensors with the largest amplitude of the summed up running standard deviations of the minima and maxima of fMCG. For example, in a given record of 10–30 min, let us assume that there are small movements in the first 10 min and in the later part of the study there are large movements. In such a case, if small movements are captured by different set of sensors as compared to the large movements, then actogramQRS approach, which uses the first three sensors with largest movements, will not identify the small movements. If one averages the summed standard deviations of the minima and maxima over all the sensors to capture these small movements, then the overall amplitude of the actogramQRS may fall to the level of the baseline even in the periods containing strong movements. From this discussion, it follows that to capture the movements reliably, one must to take into account the strength of the signal in all of the sensors. Further, instead of giving equal weights to all or a few sensors, the weight should be based on the signal strength of the sensor. One way to improve the actogramQRS approach would be to calculate the maximum of all sensors at each time point. These two properties are built-in in the actogramCOG approach by virtue of its construction and hence it is able to reliably capture the movements in the data. These enhancements in methodology along with the normalized scale (0–3 cm) of actogramCOG add the value in clearly distinguishing the movement from the baseline activity. On the other hand, large, easily perceptible fetal movements that are significant enough to generate appreciable signals in all sensors are detected equally well by both methods.
Several studies have found that the fHR and movement become more integrated with advancing gestational age, signifying the ongoing maturation and coordination of the fetal central nervous system. 8,11,26,33 Studies have also suggested that the behavioral state of the fetus is interrupted with high levels of maternal stress, preterm birth and other pregnancy high-risk conditions, which cause the de-coupling of the fetal heart and movement.8,9,19 In the future, accurate co-registration of fetal movement and heart rate performed with the methodology outlined here can be correlated to neonatal outcomes and ultimately facilitate the management of high-risk pregnancies resulting in growth-restricted or hypoxic fetuses.
CONCLUSION
Throughout pregnancy, fMCG is reliable tool to study the development of cardiac system. In addition to the well-described clinical application of using fMCG to detect cardiac anomalies, the superior spatial and temporal resolution of fMCG can be applied to the measurement of other parameters used in standard clinical practice, such as fetal movement. In this work, we have introduced a novel approach to simultaneously track the fetal movement and heart rate by constructing reliable actocardiograms in 39 fMCG data sets. The duration of the movement and its correlations with fHR acceleration detected using this approach can be used to clearly define normal values associated with the maturing fetus, and ultimately be used to enhance the predictive value of the clinical tests used to evaluate the abnormal fetus.
The proposed approach can be applied to other aspects of fetal physiology such as the understanding of the neurological maturation of the fetus using the spontaneous brain patterns and evoked response using auditory and visual modalities. One of the factors that interfere with the fetal neurological study is the fetal movement. We cannot quantify the fetal movement by using simultaneous ultrasound measurement with fMEG as it would obscure the fetal brain data and hence we developed this novel approach to track the fetal movement based on fMCG. Thus, with this new approach we can identify segments of fetal movements and exclude them from evoked response analysis. Since the heart rate is obtained naturally during the fMEG data collection, we also plan to study the fetal behavioral states by using this fetal movement detection.
ACKNOWLEDGMENTS
This work was supported by the following NIH Grants R01-EB007826 and 5R01-NS-36277.We would like to thank Jessica Temple for useful suggestions.
REFERENCES
- 1.ACOG. ACOG practice bulletin. Antepartum fetal surveillance. Number 9, October 1999 (replaces Technical Bulletin Number 188, January 1994). Clinical management guidelines for obstetrician-gynecologists. Int. J. Gynaecol. Obstet. 2000;68(2):175–185. [PubMed] [Google Scholar]
- 2.Baser I, Johnson TR, Paine LL. Coupling of fetal movement and fetal heart rate accelerations as an indicator of fetal health. Obstet. Gynecol. 1992;80(1):62–66. [PubMed] [Google Scholar]
- 3.Comani S, Liberati M, Mantini D, Gabriele E, Brisinda D, Di Luzio S, Fenici R, Romani GL. Characterization of fetal arrhythmias by means of fetal magnetocardiography in thee cases of difficult ultrasonographic imaging. Pace. 2004;27(12):1647–1655. doi: 10.1111/j.1540-8159.2004.00699.x. [DOI] [PubMed] [Google Scholar]
- 4.Comani S, Srinivasan V, Alleva G, Romani GL. Entropy-based automated classification of independent components separated from fMCG. Phys. Med. Biol. 2007;52(5):N87–N97. doi: 10.1088/0031-9155/52/5/N02. [DOI] [PubMed] [Google Scholar]
- 5.Czerwinski RN, Jones DL, O’Brien WD., Jr Detection of lines and boundaries in speckle images—application to medical ultrasound. IEEE Trans. Med. Imaging. 1999;18(2):126–136. doi: 10.1109/42.759114. [DOI] [PubMed] [Google Scholar]
- 6.de Vries JI, Visser GH, Prechtl HF. The emergence of fetal behaviour. II. Quantitative aspects. Early Hum. Dev. 1985;12(2):99–120. doi: 10.1016/0378-3782(85)90174-4. [DOI] [PubMed] [Google Scholar]
- 7.de Vries JIP. The first trimester. In: Nijhuis JG, editor. Fetal Behavior: Development and Perinatal Aspects. NY: Oxford University Press; 1992. pp. 3–16. [Google Scholar]
- 8.DiPietro JA, Hodgson DM, Costigan KA, Hilton SC, Johnson TR. Development of fetal movement–fetal heart rate coupling from 20 weeks through term. Early Hum. Dev. 1996;44(2):139–151. doi: 10.1016/0378-3782(95)01704-6. [DOI] [PubMed] [Google Scholar]
- 9.Dipietro JA, Irizarry RA, Hawkins M, Costigan KA, Pressman EK. Cross-correlation of fetal cardiac and somatic activity as an indicator of antenatal neural development. Am. J. Obstet. Gynecol. 2001;185(6):1421–1428. doi: 10.1067/mob.2001.119108. [DOI] [PubMed] [Google Scholar]
- 10.Estombelo-Montesco CA, de Araujo DB, Silva Filho AC, Moraes ER, Barros AK, Wakai RT, Baffa O. Dependent component analysis for the magnetogastrographic detection of human electrical response activity. Physiol. Meas. 2007;28(9):1029–1044. doi: 10.1088/0967-3334/28/9/005. [DOI] [PubMed] [Google Scholar]
- 11.Geue D, Van Leeuwen P, Lange S, Gronemeyer D. Automatic detection of gross fetal movements using actography based on magnetocardiography. Biomed Tech. 2007;52 CD-ROM. [Google Scholar]
- 12.Gonzalez RC, Woods RE. Digital Image Processing. Reading, NJ: Pearson Education, Inc; 2008. [Google Scholar]
- 13.Grimm B, Haueisen J, Huotilainen M, Lange S, Van Leeuwen P, Menendez T, Peters MJ, Schleussner E, Schneider U. Recommended standards for fetal magnetocardiography. Pacing Clin. Electrophysiol. 2003;26(11):2121–2126. doi: 10.1046/j.1460-9592.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- 14.Hertogs K, Roberts AB, Cooper D, Griffin DR, Campbell S. Maternal perception of fetal motor activity. Br. Med. J. 1979;2(6199):1183–1185. doi: 10.1136/bmj.2.6199.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange S, Van Leeuwen P, Schneider U, Frank B, Hoyer D, Geue D, Gronemeyer D. Heart rate features in fetal behavioural states. Early Hum. Dev. 2009;85(2):131–135. doi: 10.1016/j.earlhumdev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Lowery CL, Campbell JQ, Wilson JD, Murphy P, Preissl H, Malak SF, Eswaran H. Noninvasive antepartum recording of fetal S-T segment with a newly developed 151-channel magnetic sensor system. Am. J. Obstet. Gynecol. 2003;188(6):1491–1496. doi: 10.1067/mob.2003.367. (discussion 1496–1497) [DOI] [PubMed] [Google Scholar]
- 17.Maeda K, Iwabe T, Yoshida S, Ito T, Minagawa Y, Morokuma S, Pooh RK, Fuchiwaki T. Detailed multigrade evaluation of fetal disorders with the quantified actocardiogram. J. Perinat. Med. 2009;37(4):392–396. doi: 10.1515/JPM.2009.056. [DOI] [PubMed] [Google Scholar]
- 18.Mangesi L, Hofmeyr GJ. Fetal movement counting for assessment of fetal wellbeing. Cochrane Database Syst. Rev. 2007;(1) doi: 10.1002/14651858.CD004909.pub2. CD004909. [DOI] [PubMed] [Google Scholar]
- 19.Monk C, Sloan RP, Myers MM, Ellman L, Werner E, Jeon J, Tager F, Fifer WP. Fetal heart rate reactivity differs by women’s psychiatric status: an early marker for developmental risk? J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(3):283–290. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Neldam S. Fetal movements as an indicator of fetal well-being. Dan. Med. Bull. 1986;33(5):213–221. [PubMed] [Google Scholar]
- 21.Nijhuis JG. The third trimester. In: Nijhuis JG, editor. Fetal Behaviour: Development and Perinatal Aspects. NY: Oxford University Press; 1992. pp. 26–40. [Google Scholar]
- 22.Patrick J, Campbell K, Carmichael L, Natale R, Richardson B. Patterns of gross fetal body movements over 24-hour observation intervals during the last 10 weeks of pregnancy. Am. J. Obstet. Gynecol. 1982;142(4):363–371. doi: 10.1016/s0002-9378(16)32375-4. [DOI] [PubMed] [Google Scholar]
- 23.Schneider U, Frank B, Fiedler A, Kaehler C, Hoyer D, Liehr M, Haueisen J, Schleussner E. Human fetal heart rate variability-characteristics of autonomic regulation in the third trimester of gestation. J. Perinat. Med. 2008;36(5):433–441. doi: 10.1515/JPM.2008.059. [DOI] [PubMed] [Google Scholar]
- 24.Schneider U, Giessler F, Nowak H, Logemann T, Grimm B, Haueisen J, Schleussner E. Fetal MCG and fetal MEG measurements with a 3-channel SQUID system. Neurol. Clin. Neurophysiol. 2004;2004:65. [PubMed] [Google Scholar]
- 25.Schneider U, Schleussner E, Fiedler A, Jaekel S, Liehr M, Haueisen J, Hoyer D. Fetal heart rate variability reveals differential dynamics in the intrauterine development of the sympathetic and parasympathetic branches of the autonomic nervous system. Physiol. Meas. 2009;30(2):215–226. doi: 10.1088/0967-3334/30/2/008. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi H. Studies on cross correlation coefficient of fetal heart rate and fetal movement signals detected by ultrasonic Doppler fetal actocardiograph. Nippon Sanka Fujinka Gakkai Zasshi. 1990;42(5):443–449. [PubMed] [Google Scholar]
- 27.Ulusar UD, Govindan RB, Wilson JD, Lowery CL, Preissl H, Eswaran H. Adaptive rule based fetal QRS complex detection using Hilbert transform; Conf. Proc. IEEE Eng. Med. Biol. Soc; 2009. pp. 4666–4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Leeuwen P, Geue D, Lange S, Hatzmann W, Gronemeyer D. Changes in the frequency power spectrum of fetal heart rate in the course of pregnancy. Prenat. Diagn. 2003;23(11):909–916. doi: 10.1002/pd.723. [DOI] [PubMed] [Google Scholar]
- 29.Van Woerden EE, Van Geijn HP. Heart-rate patterns and fetal movements. In: Nijhuis JG, editor. Fetal Behaviour: Development and Perinatal Aspects. NY: Oxford University Press; 1992. pp. 42–56. [Google Scholar]
- 30.Vrba J, Robinson SE, McCubbin J, Lowery CL, Eswaran H, Wilson JD, Murphy P, Preissl H. Fetal MEG redistribution by projection operators. IEEE Trans. Biomed. Eng. 2004;51(7):1207–1218. doi: 10.1109/TBME.2004.827265. [DOI] [PubMed] [Google Scholar]
- 31.Wakai RT, Wang M, Leuthold AC, Martin CB. Foetal magnetocardiogram amplitude oscillations associated with respiratory sinus arrhythmia. Physiol. Meas. 1995;16(1):49–54. doi: 10.1088/0967-3334/16/1/006. [DOI] [PubMed] [Google Scholar]
- 32.Wilson JD, Govindan RB, Hatton JO, Lowery CL, Preissl H. Integrated approach for fetal QRS detection. IEEE Trans. Biomed. Eng. 2008;55(9):2190–2197. doi: 10.1109/TBME.2008.923916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao H, Wakai RT. Simultaneity of foetal heart rate acceleration and foetal trunk movement determined by foetal magnetocardiogram actocardiography. Phys. Med. Biol. 2002;47(5):839–846. doi: 10.1088/0031-9155/47/5/310. [DOI] [PubMed] [Google Scholar]