Abstract
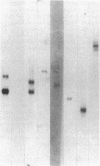
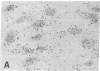
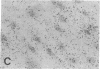
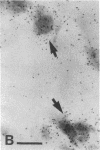
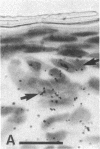
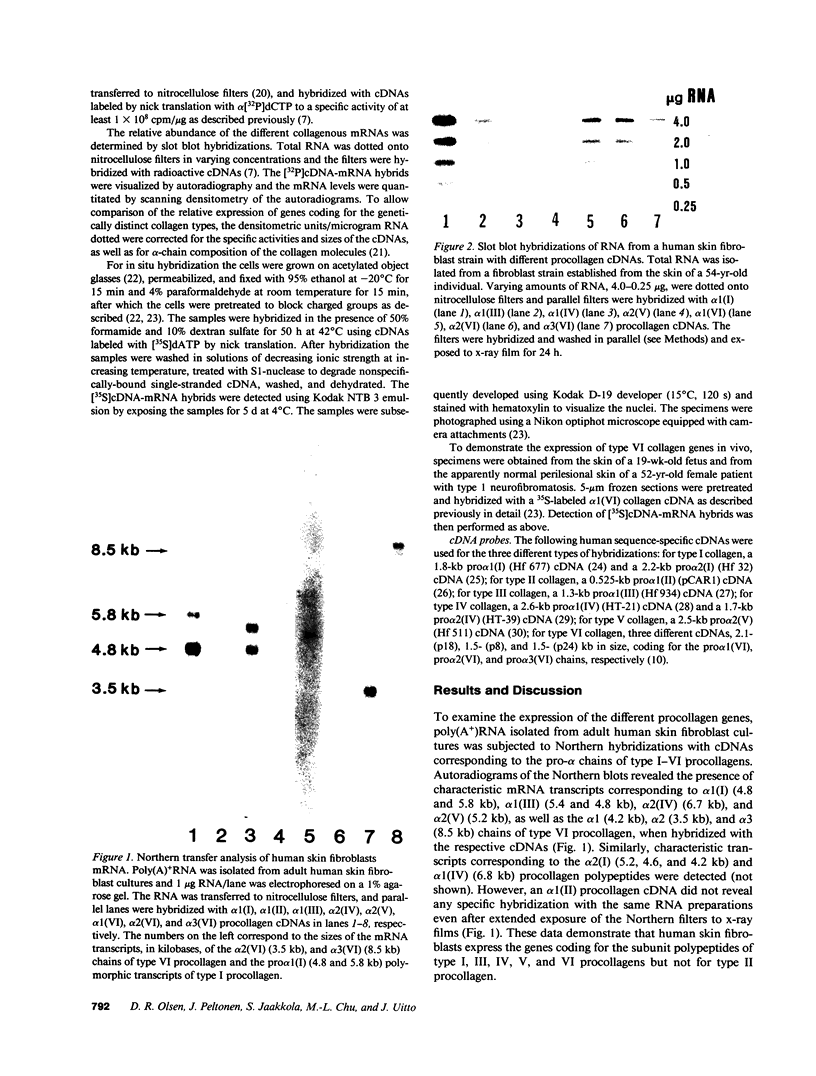
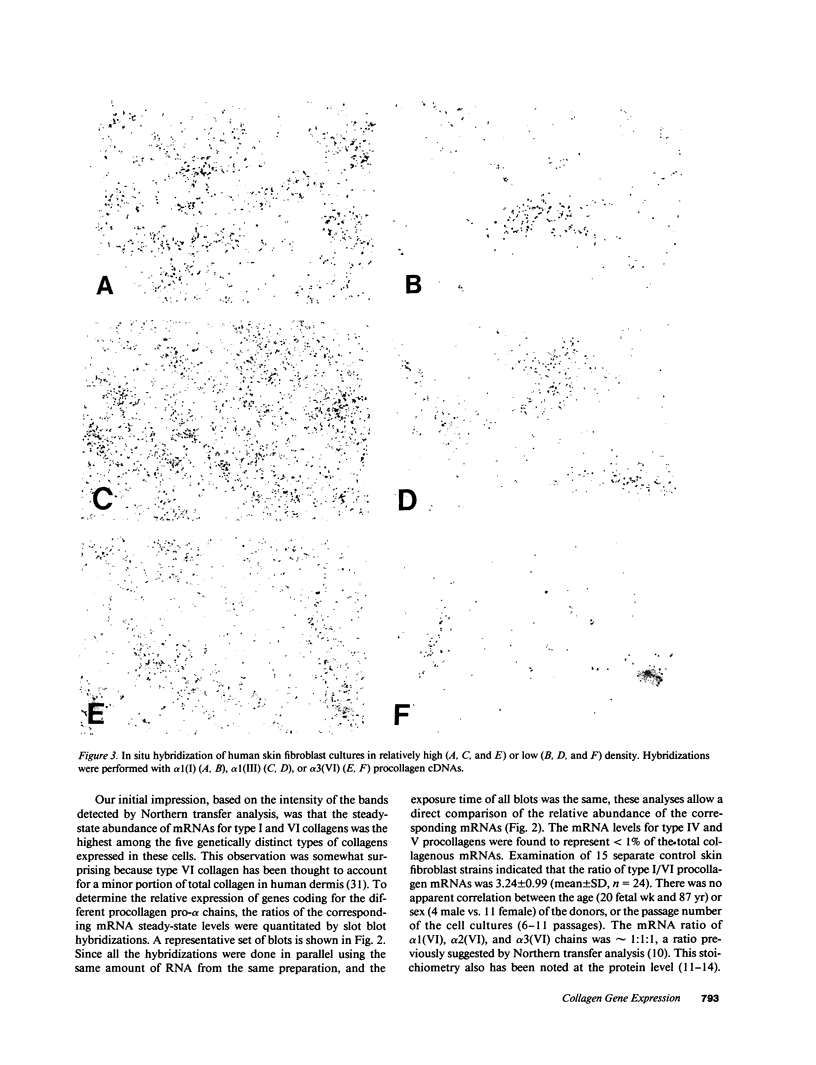
Previous studies have suggested that procollagen types I and III are the major collagenous gene products of cultured human skin fibroblasts. In this study the expression of 10 different genes, encoding the subunit polypeptides for collagen types I-VI, by human skin fibroblasts in culture was analyzed by molecular hybridizations. Northern transfer analysis demonstrated the presence of specific mRNA transcripts for collagen types I, III, IV, V, and VI, but not for type II collagen. Quantitation of the abundance of these mRNAs by slot blot hybridizations revealed that type I, III, and VI procollagens were the major collagenous gene products of skin fibroblasts in culture. The mRNAs for type IV and V collagens represented only a small percentage of the total collagenous mRNA transcripts. Further analysis by in situ hybridization demonstrated that the majority of the cultured cells coexpressed the genes for type I, III, and VI procollagen pro-alpha chains. Further in situ hybridization analyses revealed the expression of type VI collagen genes in normal human skin. These data demonstrate that human skin fibroblast cultures can be used to study the transcriptional regulation of at least nine genetically distinct procollagen genes. The data further suggest that type VI collagen, in addition to types I and III, may be a major collagenous component of human skin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K. Production of both interstitial and basement membrane procollagens by fibroblastic WI-38 cells from human embryonic lung. Biochem Biophys Res Commun. 1980 Apr 14;93(3):873–880. doi: 10.1016/0006-291x(80)91157-2. [DOI] [PubMed] [Google Scholar]
- Aumailley M., von der Mark H., Timpl R. Size and domain structure of collagen VI produced by cultured fibroblasts. FEBS Lett. 1985 Mar 25;182(2):499–502. doi: 10.1016/0014-5793(85)80362-8. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C. D., Toth-Fejel S. E., Gadi I. K., Litt M., Condon M. R., Kolbe M., Hagen I. K., Kurkinen M., Mackenzie J. W., Magenis E. The genes coding for human pro alpha 1(IV) collagen and pro alpha 2(IV) collagen are both located at the end of the long arm of chromosome 13. Am J Hum Genet. 1988 Feb;42(2):309–314. [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Mann K., Deutzmann R., Pribula-Conway D., Hsu-Chen C. C., Bernard M. P., Timpl R. Characterization of three constituent chains of collagen type VI by peptide sequences and cDNA clones. Eur J Biochem. 1987 Oct 15;168(2):309–317. doi: 10.1111/j.1432-1033.1987.tb13422.x. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Weil D., de Wet W., Bernard M., Sippola M., Ramirez F. Isolation of cDNA and genomic clones encoding human pro-alpha 1 (III) collagen. Partial characterization of the 3' end region of the gene. J Biol Chem. 1985 Apr 10;260(7):4357–4363. [PubMed] [Google Scholar]
- Colombatti A., Bonaldo P., Ainger K., Bressan G. M., Volpin D. Biosynthesis of chick type VI collagen. I. Intracellular assembly and molecular structure. J Biol Chem. 1987 Oct 25;262(30):14454–14460. [PubMed] [Google Scholar]
- Crawford S. W., Featherstone J. A., Holbrook K., Yong S. L., Bornstein P., Sage H. Characterization of a type VI collagen-related Mr-140 000 protein from cutis-laxa fibroblasts in culture. Biochem J. 1985 Apr 15;227(2):491–502. doi: 10.1042/bj2270491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Hessle H., Klier G. Molecular assembly, secretion, and matrix deposition of type VI collagen. J Cell Biol. 1986 Mar;102(3):703–710. doi: 10.1083/jcb.102.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay S., Martin G. R., Muller P. K., Timpl R., Kuhn K. Simultaneous synthesis of types I and III collagen by fibroblasts in culture. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4037–4040. doi: 10.1073/pnas.73.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessle H., Engvall E. Type VI collagen. Studies on its localization, structure, and biosynthetic form with monoclonal antibodies. J Biol Chem. 1984 Mar 25;259(6):3955–3961. [PubMed] [Google Scholar]
- Murata K., Motoyama T., Suka M., Ohno M., Kuboki Y. High production of type VI collagen in multiple fibromatosis with multiple articular dysplasia. Biochem Biophys Res Commun. 1987 Aug 31;147(1):275–281. doi: 10.1016/s0006-291x(87)80117-1. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Chu M. L., Faro S. H., Clark W. J., Prockop D. J., Ramirez F. Cloning a cDNA for the pro-alpha 2 chain of human type I collagen. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3516–3520. doi: 10.1073/pnas.78.6.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Howard P. S., Jelen A. M., Dion A. S., Macarak E. J. Duplication of type IV collagen COOH-terminal repeats and species-specific expression of alpha 1(IV) and alpha 2(IV) collagen genes. J Biol Chem. 1987 Jul 5;262(19):9231–9238. [PubMed] [Google Scholar]
- Myers J. C., Loidl H. R., Seyer J. M., Dion A. S. Complete primary structure of the human alpha 2 type V procollagen COOH-terminal propeptide. J Biol Chem. 1985 Sep 15;260(20):11216–11222. [PubMed] [Google Scholar]
- Narayanan A. S., Page R. C. Biosynthesis and regulation of type V collagen in diploid human fibroblasts. J Biol Chem. 1983 Oct 10;258(19):11694–11699. [PubMed] [Google Scholar]
- Narayanan A. S., Page R. C. Synthesis of type V collagen by fibroblasts derived from normal, inflamed and hyperplastic human connective tissues. Coll Relat Res. 1985 Sep;5(4):297–304. doi: 10.1016/s0174-173x(85)80019-4. [DOI] [PubMed] [Google Scholar]
- Ohta A., Uitto J. Procollagen gene expression by scleroderma fibroblasts in culture. Inhibition of collagen production and reduction of pro alpha 1(I) and pro alpha 1(III) collagen messenger RNA steady-state levels by retinoids. Arthritis Rheum. 1987 Apr;30(4):404–411. doi: 10.1002/art.1780300407. [DOI] [PubMed] [Google Scholar]
- Olsen D. R., Chu M. L., Uitto J. Expression of basement membrane zone genes coding for type IV procollagen and laminin by human skin fibroblasts in vitro: elevated alpha 1 (IV) collagen mRNA levels in lipoid proteinosis. J Invest Dermatol. 1988 May;90(5):734–738. doi: 10.1111/1523-1747.ep12560934. [DOI] [PubMed] [Google Scholar]
- Peltonen J., Jaakkola S., Lebwohl M., Renvall S., Risteli L., Virtanen I., Uitto J. Cellular differentiation and expression of matrix genes in type 1 neurofibromatosis. Lab Invest. 1988 Dec;59(6):760–771. [PubMed] [Google Scholar]
- Pihlajaniemi T., Tryggvason K., Myers J. C., Kurkinen M., Lebo R., Cheung M. C., Prockop D. J., Boyd C. D. cDNA clones coding for the pro-alpha1(IV) chain of human type IV procollagen reveal an unusual homology of amino acid sequences in two halves of the carboxyl-terminal domain. J Biol Chem. 1985 Jun 25;260(12):7681–7687. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trüeb B., Schreier T., Bruckner P., Winterhalter K. H. Type VI collagen represents a major fraction of connective tissue collagens. Eur J Biochem. 1987 Aug 3;166(3):699–703. doi: 10.1111/j.1432-1033.1987.tb13568.x. [DOI] [PubMed] [Google Scholar]
- Trüeb B., Winterhalter K. H. Type VI collagen is composed of a 200 kd subunit and two 140 kd subunits. EMBO J. 1986 Nov;5(11):2815–2819. doi: 10.1002/j.1460-2075.1986.tb04573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorio E., Sandell L., Kravis D., Sheffield V. C., Vuorio T., Dorfman A., Upholt W. B. Construction and partial characterization of two recombinant cDNA clones for procollagen from chicken cartilage. Nucleic Acids Res. 1982 Feb 25;10(4):1175–1192. doi: 10.1093/nar/10.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D., Bernard M., Gargano S., Ramirez F. The pro alpha 2(V) collagen gene is evolutionarily related to the major fibrillar-forming collagens. Nucleic Acids Res. 1987 Jan 12;15(1):181–198. doi: 10.1093/nar/15.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D., Mattei M. G., Passage E., N'Guyen V. C., Pribula-Conway D., Mann K., Deutzmann R., Timpl R., Chu M. L. Cloning and chromosomal localization of human genes encoding the three chains of type VI collagen. Am J Hum Genet. 1988 Mar;42(3):435–445. [PMC free article] [PubMed] [Google Scholar]