Abstract
Genetic analysis of the Drosophila leg-arista-wing complex (lawc) gene suggests a role for the Lawc protein in chromatin-related processes based on its classification as a trxG gene but the molecular mechanisms of its function remain elusive. We have found that Lawc is a small, cysteine-rich protein that is present in most of the interbands of polytene chromosomes. In agreement with this observation, Lawc co-localizes with RNA polymerase IIo (Pol IIo) and it is recruited to transcribed loci after elongation by Pol IIo has begun. Lawc interacts with the nuclear proteasome regulator dREGγ in a yeast two-hybrid assay and both proteins co-localize on polytene chromosomes. In addition, a mutation in lawc interacts genetically with a mutation in a component of the proteasome. lawc mutants show decreased expression of some genes, while the levels of Pol IIoSer2 increase. We conclude that Lawc is required for proper transcription by RNA polymerase II in a process that involves the nuclear proteasome.
Keywords: transcription, Lawc, RNA polymerase II, dREGγ, proteasome
INTRODUCTION
Transcription by RNA polymerase II (Pol II) is a tightly regulated and complex process designed to allow precise control of gene expression in eukaryotes. The first step in transcription by Pol II is the formation of the preinitiation complex. Sequence-specific activators bind to DNA elements near the gene and recruit ATP-dependent chromatin remodeling complexes, histone-modifying enzymes, and/or components of the mediator scaffold complex to serve as coactivators. Coactivators ultimately operate to make promoter-proximal chromatin accessible to additional factors required for transcription, including unphosphorylated Pol II (Pol IIa), as part of the preinitiation complex (Lu et al. 1991). The steady-state levels of transcription regulators at the promoter can be controlled by recruitment or by degradation. Here we explore the possibility that a new protein named Lawc is involved in regulating the activity of the REGγ proteasome to control transcription elongation.
During transcription, the recruitment of Pol IIa is followed by promoter melting, transcription initiation, and promoter clearance. The last component of the preinitiation complex to be recruited, TFIIH, can both unwind the DNA template and phosphorylate the carboxy-terminal domain (CTD) of the largest Pol IIa subunit on serine 5 of the heptapeptide repeat (Pol IIoSer5) (Lu et al. 1992; Schaeffer et al. 1993; Schwartz et al. 2003). CTD phosphorylation may disrupt contacts between Pol II and proteins involved in preinitiation; the Pol II complex can then move away from the promoter and transcription can progress. In addition, Pol IIoSer5 recruits, and sometimes stimulates, factors needed for initiation and processing of the 5′ end of the transcript, such as RNA-capping enzyme (Ho et al. 1999; Schroeder et al. 2000). DSIF and NELF proteins mediate promoter proximal pausing just downstream of the transcription start site (Wu et al. 2005; Yamaguchi et al. 1999). The significance of this stage is not fully understood, but it may serve as a checkpoint for efficient mRNA capping. Next Pol IIoSer5 is phosphorylated on CTD serine 2 (Pol IIoSer2) by p-TEFb and the pause is released so that Pol IIo can continue along the template in the elongation phase (Marshall et al. 1996).
The 26S proteasome also plays a role in transcription. This proteasome is both nuclear and cytoplasmic, and consists of a 20S catalytic core and two 19S lid/base subunits. A 19S subunit can recognize ubiquinated substrates and funnel them into the core to be degraded (reviewed in Kinyamu et al. 2005). The 19S subunit and the 20S core have been detected at active genes and 19S seems to be involved in elongation (Ferdous et al. 2001; Gillette et al. 2004; Gonzalez et al. 2002). Moreover, the proteasome is required for transcriptional activation by many members of the Nuclear Hormone Receptor (NHR) family of transcription factors. It seems that the order of recruitment of coactivators to genes targeted by NHRs is important and that proteasome-mediated degradation of some NHRs and corepressors is required for proper transition between various complexes and successful transcription (Dennis et al. 2005; Reid et al. 2003).
Transcription of genes in response to hormones by activated class II NHRs has been extensively studied (reviewed in King-Jones et al. 2005). These NHRs bind to DNA and serve as transcriptional repressors in the absence of their ligand via corepressor recruitment. However, they bind DNA and become activators in the presence of the hormone ligand, usually in the form of a heterodimer. One well-studied class II NHR is the Drosophila Ecdysone Receptor (EcR). Drosophila chromosomes have many puff sites induced by the 20 hydroxyecdysone steroid (20E). Genes activated by 20E are involved in the development of the fly as it transitions from larva to adult. This activation is mediated by the EcR when bound to 20E and Ultraspiracle by recruiting coactivators such as Tai (Bai et al. 2000; Yao et al. 1993; Yao et al. 1992). The REGγ proteasome is a conserved nuclear complex consisting of the 20S core and 11S subunits. The 11S subunit is a REGγ homoheptamer and it substitutes for the 19S lid/base to degrade proteins in an ATP and ubiquitin-independent manner (Dubiel et al. 1992; Masson et al. 2001; Wilk et al. 2000). One of the few known targets of the REGγ proteasome is the SRC-3 coactivator, which is the mammalian homologue of the Drosophila Tai protein (Bai et al. 2000; Li et al. 2006).
Mutants in the Drosophila melanogaster leg-arista-wing complex (lawc) gene have been previously isolated and characterized. Flies carrying a mutation in lawc have partial arista-to-leg transformations and the Drosophila lawc gene was classified as a member of the trxG (Zorin et al. 1999). Given this link between lawc and chromatin, we decided to analyze the role of the Lawc protein in chromatin structure and transcription. Here we find that Lawc is a novel, small, cysteine-rich nuclear protein that is present at most of the interbands of polytene chromosomes. Lawc co-localizes with Pol IIo at numerous chromosomal sites where it appears to be recruited after elongation by Pol IIo has begun. lawc mutants show an increase in the phosphorylated Pol IIoSer2 form of RNA polymerase II. Nevertheless, some genes show decreased expression in lawc mutants, suggesting that the increase in Pol IIoSer2 is not always productive. Lawc interacts with the nuclear proteasome regulator dREGγ in a yeast-two-hybrid screen and lawc interacts genetically with a mutation in a component of the proteasome core. We conclude that Lawc is required for proper transcription by RNA polymerase II in a process that involves the nuclear proteasome.
MATERIALS AND METHODS
Fly strains and construction of transgenic flies
Fly stocks were maintained in standard medium at 25°. Transgenic flies were obtained by P element-mediated transformation. A 6.5 kb fragment of DNA containing the coding region of CG32711 plus 3,987 bp of 5′ and 447 bp of 3′ sequence was obtained by PCR of genomic DNA and cloned into the pCaSpeR vector. The phenotype of y w ctn lawcP1 females was then compared to that of y w ctn lawcP1 females homozygous for the transgene. pros26 pb pp/TM3 Sb Ser and pb pp/TM3 Sb Ser fly stocks were obtained from the Bloomington Stock Center. wa ctn lawcP1/FM7i flies were crossed to test for phenotypic enhancement/suppression and wa ctn/FM7i was used in control crosses. The Chi squared test was used to determine the statistical significance of phenotypic enhancement or suppression.
Real-time RT-PCR
RNA was extracted from five male third instar larvae per genotype (wa ctn lawcP1 and the wa ctn control) using the RNA Bee isolation solution and protocol (AMS Biotechnology). RNA from third instar salivary glands of the above genotypes (40 pairs per genotype) was isolated using the QIAshredder columns and RNeasy Micro kit (Qiagen). RNA quantity was calculated spectrophotometricaly. Two-step real-time RT-PCR was performed using QuantiTect kits (Qiagen) and a Bio-Rad iCYCLER iQ as instructed by manuals. QuantiTect primer assays were used: Dm_Act5C_1_SG , Dm_CG32711_1_SG, Dm_Trf2_1_SG, Dm_CG11190_1_SG, Dm_Act88F_1_SG, Dm_Hsp70Aa_1_SG, Dm_ACXD_1_SG, and Dm_CG9497_2_SG (Qiagen). Primer efficiency was verified on PCR products cloned into pCR2.1. For comparing control and experimental transcript levels, Cycle threshold (Ct) levels of the transcript under investigation were subtracted from the Act5C Ct (ΔCt). The Student's T-Test was used to determine statistical significance of ΔCt values. One control larva was set as the reference, and then ΔΔCt values for each larva were calculated and averaged to generate figure graphs.
Protein purification and antibody production and western analysis
The Lawc open reading frame was amplified by PCR and cloned into the pET100D-TOPO vector in-frame to the N-terminal His6 tag. Cultures of His-Lawc in the BL21 bacterial strain were induced with 1 mM IPTG, grown for 2.5 hr, lysed under denaturing conditions, and purified by Ni chromatography. The protein was then concentrated in a Centricon YM-10 (Millipore) and dialyzed into PBS via a 10,000 MWCO Slide-A-Lyzer dialysis cassette (Pierce). Purified His-Lawc was used to immunize rabbits by standard procedures (PRF&L). The specificity of the antisera versus that of the preimmune was verified by Western analysis of larval and His-Lawc-expressing bacterial protein extracts and by immunofluorescence microscopy using polytene chromosomes. For western analysis, third instar larvae (five per genotype) were homogenized in protein sample buffer (PSB): 0.25 M Tris-HCl pH 6.8, 0.063 M Tris, 10% glycerol, 2% SDS, 0.0025% bromophenol blue, 50 mM DTT, 1X cOmplete, mini protease inhibitor (Roche), and 1 mM PMSF. For western analysis of Pol IIo, the PSB also contained 1:100 phosphatase inhibitor solution (Sigma) and 5 mM β-glycerophosphate. Extracts were run on Novex Tris-Glycine gels (4-20% for Lawc westerns and 4-12% for Pol IIo westerns) (Invitrogen). Gels were then transfered to 0.45 μm PVDF membranes in CAPS buffer (10 mM CAPS free acid, 3 mM DTT, 15% methanol, pH 10.5). Membranes were stained with Ponceau to confirm adequate transfer, destained in CAPS, blocked in PBS containing 5% milk and 0.25% Tween for 30 min, and exposed to the primary antibody overnight at 4°. Subsequently, the membrane was washed in PBS, 0.25% Tween, incubated with secondary antibody for 1 hr at room temperature, and then washed again. Lastly, SuperSignal West Pico chemiluminescent substrate (Pierce) was added and detected on film. The following antibody dilutions were used: 1:1,000 rabbit α-Lawc, 1:5,000 rabbit α-Lamin, 1:500 H5 (Covance), 1:500 H14 (Covance), and 1:10,000 for all secondary antibodies (HRP α-rabbit and HRP α-mouse IgM).
Immunohistochemistry and fluorescent in situ hybridization
Wild-type and wa ctn third instar larvae were dissected and polytene chromosome were fixed and squashed as previously described (Ivaldi et al. 2007) with the following exceptions: the fix contained 50% acetic acid, 2% formaldehyde, and 0.1% Triton X-100, slides were processed immediately (not stored), in some instances, the antibody dilution buffer contained dry milk rather than BSA, a blocking step prior to antibody incubation was performed two times for 30 min, wash steps were performed with PBS, 0.1% Tween, and the DAPI concentration was 0.06 μg/mL. The following antibody dilutions were used: 1:20 or 1:30 rabbit α-Lawc, 1:150 rabbit α-dREGγ (Masson et al. 2001), 1:75 H5 (Covance), 1:75 H14 (Covance), 1:5 mouse α-EcR (Developmental Studies Hybridoma Bank) (Talbot et al. 1993), and 1:250 for all secondary antibodies (Texas Red α-rabbit, FITC α-rabbit, FITC α-mouse IgM, and Texas Red α-mouse IgG). The fluorescent in situ hybridization (FISH) portion of the combined FISH and immunofluorescence on polytene chromosomes was performed as previously described (Ivaldi et al. 2007) with the following exceptions: freshly squashed slides were left out 1 hr at room temperature before freezing and probes were denatured at 65°. The subsequent immunohistochemistry was performed as described above except that the primary antibody was incubated 2.5 hr at 37°.
Yeast two-hybrid screen
The yeast two-hybrid screen was carried out with the lawc ORF as previously reported (Capelson et al. 2005) with the following exceptions: western blot analysis confirmed the expression of lawc in the yeast and more than six positive clones were selected for sequencing.
RESULTS
lawc corresponds to a previously uncharacterized gene
The Drosophila melanogaster lawc gene was identified based on the mutant phenotype caused by a P-element insertion between 7D10 and 7F1, generating lawcP1 (Zorin et al. 1999). This mutant phenotype consists of an arista-to-leg transformation and an enhancement of the cut wing phenotype of ctn. We noted that this allele is semilethal, with about 35-65% of lawcP1 mutant larvae surviving to adulthood (data not shown), and it is therefore likely that the allele is hypomorphic. Excision of the P element reverts the mutant phenotype to wild type, indicating that the mutation is caused by the presence of the P element (Zorin et al. 1999). The P-element insertion site in lawcP1 was mapped 400 bp upstream of the previously uncharacterized gene CG32711 (I. Zorin and T. Brandt, unpublished data). The UCSC genome browser (http://genome.ucsc.edu/) was used to determine that CG32711 is conserved among Drosophila species but we were unable to find a CG32711 homologue outside of Drosophila (data not shown) (Kent et al. 2002). CG32711 has an unusual structure. The open reading frame is very short, encoding only 73 amino acids, and the termination codon is located in the first of four exons (Crosby et al. 2007). It is therefore possible that the apparent absence of a lawc gene homologue from the genome of other higher eukaryotes could be due to incomplete annotation of small genes in these genomes.
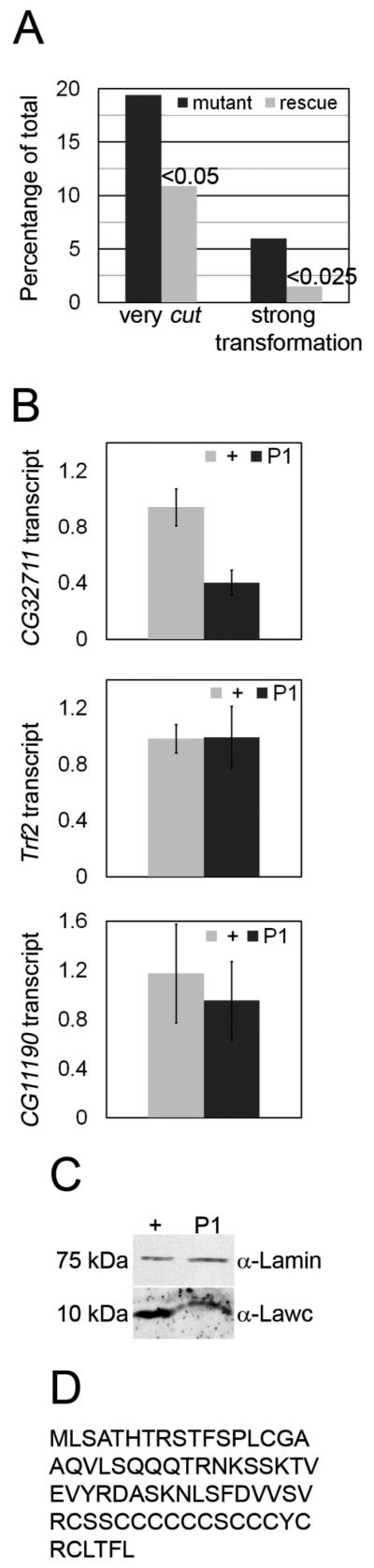
In order to test whether CG32711 corresponds to lawc, we introduced this gene into the Drosophila genome by P element-mediated transformation. Although CG32711 has a very large 5′ regulatory region, we were able to almost completely rescue the lawcP1 mutant phenotype with a transgenic construct containing the CG32711 coding sequence plus 3,987 bp of 5′ and 447 bp of 3′ sequences (Figure 1A). lawcP1 flies carrying the CG32711 transgene show a 75% reduction in the frequency of strong arista-to-leg transformations (α < 0.025) and a 44% reduction in the frequency of very cut wings (α < 0.05). In addition, the transgene also rescues the larval/pupal lethality of the lawcP1 allele (data not shown). The failure to completely rescue the lawcP1 phenotype may be due to the absence of regulatory sequences required for proper expression of CG32711 located outside of the region used in the transgene.
Figure 1.
The lawc gene corresponds to CG32711. (A) Wing and arista phenotypes of ctn lawcP1 (mutant) and ctn lawcP1; P[CG32711 rescue construct] (rescue) flies are shown with statistical significance represented by α values indicated above each rescue bar. (B) Transcript levels obtained by qRT-PCR from three separate larval extracts of each genotype (hemizygous ctn (+) and hemizygous ctn lawcP1 (P1)) were normalized to Act5C. One + extract was set as the reference and ΔΔCt values for each extract were calculated and then averaged. Graphs report results with C32711, Trf2, and CG11190 primers. (C) Western analysis of ctn (+) and ctn lawcP1 (P1) third instar larvae using α-Lawc antibodies confirms the reduction in CG32711/lawc gene expression detected by qRT-PCR. α-Lamin was used as a loading control. (D) Predicted amino acid sequence of the Lawc protein.
To further test the hypothesis that the P-element in lawcP1 affects the expression of the CG32711 gene, transcript levels in ctn lawcP1 larvae were measured by quantitative real-time RT-PCR (qRT-PCR) and compared to transcript levels in control ctn larvae. RNA was extracted from five larvae per genotype and qRT-PCR experiments were run in triplicate. Transcript levels were normalized to Actin 5C mRNA. lawcP1 mutant larvae have 58% less CG32711 transcript than the control (α < 0.004), while transcription of the gene immediately upstream, Trf2, and the gene immediately downstream, CG11190, remain unaffected (Figure 1B). These results were reproducible and similar results were obtained normalizing CG32711 transcript levels to Act88F (data not shown). In order to determine whether the levels of the CG32711 protein are also affected by mutations in the lawc gene, an antibody was created against the predicted protein product of CG32711. This antibody detects a band of approximately 10 kDa on western blots of larval protein extracts (Figure 1C). This molecular weight is very close to the predicted size of the CG32711 protein. Importantly, this band is not recognized by preimmune serum (data not shown). We observed a reduction in the CG32711 protein in ctn lawcP1 larvae relative to ctn larvae used as a control, confirming that lawc is CG32711 (Figure 1C).
Lawc localizes to the interbands of polytene chromosomes and partially co-localizes with Pol IIo
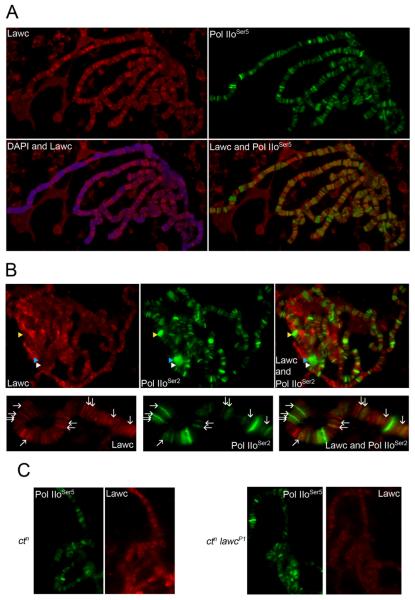
In order to gain additional insights into the possible role of Lawc in chromatin structure and gene regulation, we determined its distribution on polytene chromosomes by immunofluorescence microscopy. Antibodies against Lawc detect a protein present at nearly every interband on polytene chromosomes, including puffs (Figure 2A), whereas the preimmune serum does not recognize any protein (data not shown). Interbands are regions of open chromatin and contain genes that are actively transcribed in polytene cells as well as Pol IIo phosphorylated in the Ser2 and Ser5 residues of the CTD repeat. Lawc partially co-localizes with Pol IIoSer5 and Pol IIoSer2 (Figure 2A-B). A reduction of Lawc protein was observed in chromosomes from lawcP1 mutant larvae but levels of Pol IIoSer5 appear to be unaffected (Figure 2C). Although Lawc co-localizes with the initiating/early elongating form of Pol II (Pol IIoSer5) quite well, we noted that the intensity of Lawc signal on chromosomes often does not correlate with the intensity of Pol IIo at the same location. This tendency is more apparent when the co-localization between Lawc and elongating Pol IIoSer2 is closely examined. Lawc seems to co-localize with faint Pol IIoSer2 bands best. Bright Pol IIoSer2 bands tend to correspond to regions containing little or no Lawc protein. Nevertheless, the co-localization between active Pol II and Lawc suggest an involvement of this protein in some aspect of the transcription process.
Figure 2.
Lawc localizes to the interbands of polytene chromosomes and partially co-localizes with Pol IIo. (A) Polytene chromosomes from wild-type third instar larvae were incubated with α-Lawc, α-Pol IIoSer5 (H14) and and DAPI (blue). (B) Polytene chromosomes from wild-type third instar larvae were incubated with α-Lawc and α-Pol IIoSer2 (H5). Arrowheads point to 74EF (white), 75B (blue), and 78D (yellow) puffs. In order to allow for a more detailed examination of α-Lawc and H5 staining patterns, an enlarged and rotated version of a portion of a chromosome from each image is represented immediately below the image of the full spread. Arrows indicate some regions of Lawc-Pol IIoSer2 co-localization. (C) Polytene chromosomes from ctn and ctn lawcP1 larvae incubated with α-Lawc and α-Pol IIoSer5 antibodies.
Lawc is recruited to puffs after transcription has begun
Lawc is present at the 74EF and 75B ecdysone puffs but does not co-localize completely with active Pol IIo (Figures 2A and 2B). To address this apparent discrepancy, we considered the possibility that Lawc is recruited to active genes at a precise stage during the transcription process, and that loci at which active Pol IIo and Lawc fail to co-localize are in a different stage of the transcription cycle. To test this hypothesis, we took advantage of the fact that transcription of certain 20E-responsive genes has been extensively characterized and the timing of transcription at these loci can be examined in polytene chromosomes. We used puff staging to determine the transcriptional status of genes at various 20E-responsive loci (Ashburner 1972; Dworniczak et al. 1983). There are three types of ecdysone puffs: intermolt, early, and late. At puff stage (PS) 1, the intermolt puffs are visible. At this time, a 20E pulse occurs, which will turn intermolt genes off and early genes on. Consequently, at PS 2 the intermolt puffs are no longer visible and the first sign of puffing at early gene loci can be observed. Eventually the early genes become fully active and the larva has large puffs at early gene loci, but no late puffs; this corresponds to PS 3-5. The Ashburner model proposes that products of the early genes provide negative feedback to inactivate early genes and, in addition, activate the late genes (Ashburner 1990). Therefore, when puffs are present at both early and late gene loci, the early genes are in the process of turning off while the late genes have just been activated (PS 6-8). Visible early and late puffs allow for the identification of a PS 6-8 larva and provide a way to observe how chromatin at early gene loci is changing in response to transcriptional cessation. The presence of only late puffs indicates that the larva is in PS 9-10.
The 74EF early puff contains two 20E-responsive isoforms: E74A and the shorter E74B. E74A is an early gene and E74B can be considered an intermolt gene (Huet et al. 1993; Karim et al. 1992). While some transcript from E74A can be detected at PS 2, transcript levels seem to plateau at PS 4, when 74EF puff size peaks, at levels well over two-fold that of PS 2 (Huet et al. 1993; Karim et al. 1992). The 75B early puff has two early gene isoforms, E75A and the shorter, less abundant E75B, and one intermolt isoform, E75C. The E75C transcript is barely detectable, even at PS 1, while the E75A early transcript shows the same transcription dynamics as E74A (Huet et al. 1993; Karim et al. 1992).
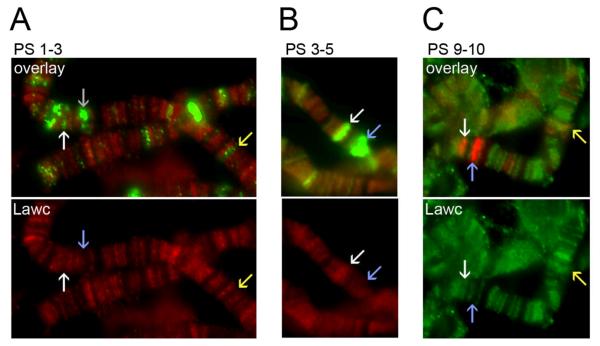
We examined the presence of Lawc at 20E-responsive loci during various puff stages. We find Lawc to be absent from the early puff (74EF and 75B) and late puff loci (such as 78D) at PS 1-3 while some Pol IIoSer2 is present (Figure 3A). Lawc and Pol IIoSer5 are present in early puffs at PS 3-5 (Figure 3B), suggesting that Lawc is present at 20E-responsive loci during the transcriptional peak of E74A and E75A and, therefore, that the recruitment of Lawc is downstream of at least one round of transcription by Pol IIo. Although Lawc is present during PS 3-5, we noted that the α-Lawc signal is not always intense (Figure 3B). After PS8, the early genes are repressed by the EcR and neither Lawc nor Pol IIoSer2 are present (Figure 3C and data not shown). Consistent with the timing observed at early puffs, Lawc is at the visibly open late puff 78D after PS 8 (Figure 3C).
Figure 3.
Lawc is recruited to ecdysone puffs after transcription has begun. (A, B, and C) Polytene chromosomes from wild-type third instar larvae were incubated with α-Lawc and (A) α-Pol IIoSer2 (H5) (green), (B) H14 (green), or (C) α-EcR (red). The PS of each larva was determined as described in the text. The white arrows point to 74EF, the blue arrows indicate 75B and the yellow arrows point to 78D.
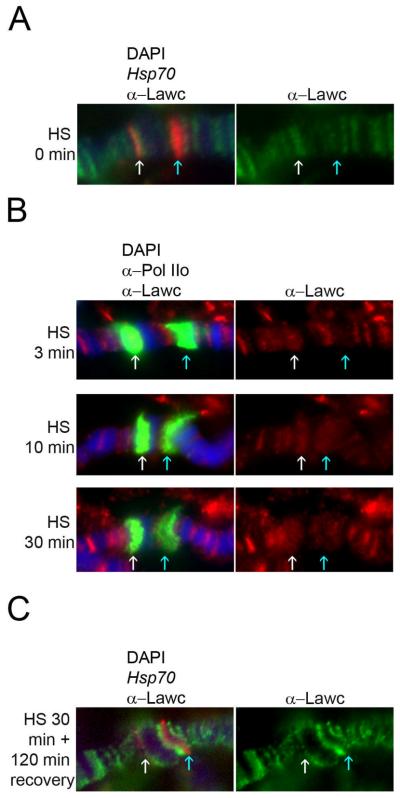
To examine whether this temporal recruitment of Lawc is general or specific to ecdysone puffs, we studied the distribution of Lawc in relation to the formation of heat-shock puffs. These puffs are located at 87A and 87C, they contain multiple copies of the Hsp70 heat-shock protein-coding gene, and they form shortly after activation by heat shock (37°). Combined fluorescent in situ hybridization (FISH) and immunofluorescence was used to demonstrate that Lawc is not present at the 87C locus before heat shock (Figure 4A). It is, however, present at 87A (Figure 4A). It has been demonstrated that, while there is no Pol IIoSer2 at 87C prior to heat shock, Pol IIoSer2 is present at 87A (Ivaldi et al. 2007). Therefore, it is likely that there is another gene at or near 87A that is being transcribed before heat shock and it may be more appropriate to make conclusions based on the heat-shock response at 87C. Strong transcriptional induction of the Drosophila Hsp70 genes is rapid following exposure to elevated temperatures. In just two minutes of heat-shock treatment, the amount of Hsp70 transcript is increased about 150-fold in larvae (Zhao et al. 2006). After three minutes of heat shock, the Hsp70 genes residing at 87C are active but Lawc is not present (Figure 4B). However, after ten minutes, Lawc is also present at the heat-shock puffs (Figure 4B). Ten minutes corresponds to the peak of Pol IIo recruitment (Yao et al. 2006). FISH experiments demonstrate that once 87C has recovered from heat shock, Lawc is not present at the locus (Figure 4C). The timing of Lawc recruitment at heat-shock genes seems very similar to that of 20E-responsive genes: Lawc is not present before or immediately following transcription initiation, becomes abundant after at least one full round of transcription, and disappears when transcription has ceased.
Figure 4.
Lawc is recruited to heat-shock puffs after transcription has begun. Images are shown with 87A on the left (white arrow) and 87C on the right (blue arrow). (A) Combined FISH-immunofluorescence was performed on wild-type polytene chromosomes with DAPI (blue), an Hsp70 probe (red), and α-Lawc. (B) Wild-type larvae were placed at 37° (HS) for the indicated amount of time. Polytene chromosomes were incubated with DAPI (blue), α-Lawc, and α-Pol IIo (green). (C) The experiment was carried out as in (A) except larvae were exposed to 37° and then allowed to recover at 25°.
The amount of Pol IIoSer2 increases in the lawcP1 mutant but transcript levels do not
The results discussed above suggest that Lawc is recruited after genes are transcriptionally active and at the peak of RNA production. To further examine the role of Lawc in the transcription process, we determined the levels of initiating Pol IIo (Pol IIoSer5) and elongating Pol IIo (Pol IIoSer2) in the lawcP1 mutant by western analysis. The results show that lawc mutants have a higher amount of Pol IIoSer2 than the control, while Pol IIoSer5 remains unaffected (Figure 5A). Since Lawc co-localizes with Pol IIo, responds to changes in the transcriptional status of 20E and heat shock-responsive genes, and has an effect on phosphorylation of the CTD of Pol IIo, we conclude that Lawc is involved in some aspect of the transcription process, although it is not clear that the increase in Pol IIoSer2 accumulation actually results in productive transcription.
Figure 5.
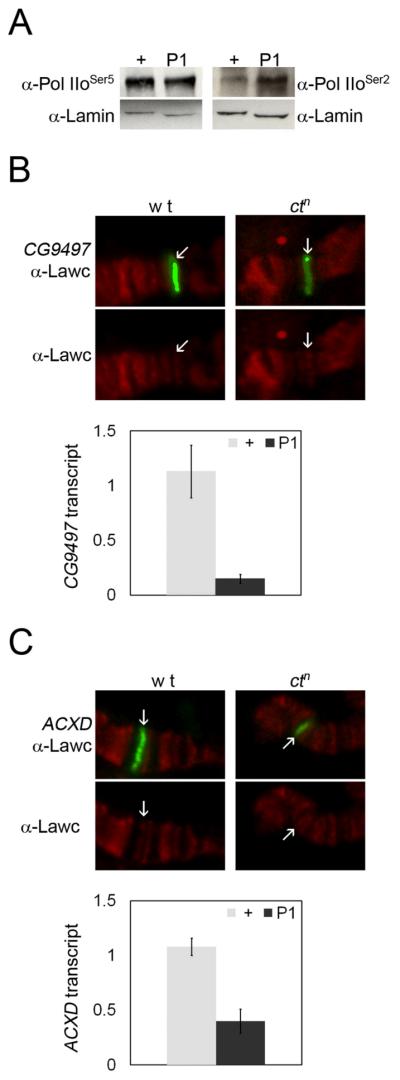
The amount of Pol IIoSer2 is augmented in the lawcP1 mutant but transcript levels are not. (A) Western analysis on hemizygous ctn (+) and hemizygous ctn lawcP1 (P1) third instar larvae with H14 and H5 antibodies shows an increase of the Pol IIoSer2 form in the ctn lawcP1 mutants. α-Lamin was used as a loading control. (B and C) Combined FISH-immunofluorescence with α-Lawc was performed on polytene chromosomes from wild-type male third instar larvae (wt) or ctn third instar male larvae. FISH probes (green) were made to either (B) CG9497 or (C) ACXD. Graphs depict the result of qRT-PCR experiments comparing (B) CG9497 and (C) ACXD transcript levels in hemizygous ctn (+) and hemizygous ctn lawcP1 (P1) third instar larval salivary glands. Relative transcript levels are calculated as described for Figure 1.
To address this issue, we began a search for genes whose expression is altered in the lawcP1 mutant background. Although it is possible that genes at 75B or 74EF are affected, these genes would be difficult to analyze in lawc mutants because puff stages progress quickly and the expression of these genes could not be directly compared among larvae unless the animals were puff staged cytogenetically, which would make the isolation of enough starting material for gene expression analysis very laborious. However, the effect of the lawcP1 mutation on Hsp70 transcription is testable. qRT-PCR was performed with Hsp70 primers on mutant and control larvae incubated at 37° for 20 or 60 min. We were unable to detect a difference during either of these conditions (data not shown). Since lawcP1 does not affect Hsp70 transcription, we carried out microarray analysis to examine differences in transcription between the ctn lawcP1 mutant and the ctn control. Several genes were then selected for further testing.
Alterations in gene expression in ctn lawcP1 mutants could be a direct effect of the absence of Lawc protein or an indirect effect caused by changes in the levels of Lawc-regulated proteins. Therefore, we first performed combined fluorescent in situ hybridization (FISH) and immunofluorescence analysis using Lawc antibodies on polytene chromosomes from wild-type and ctn larvae to eliminate genes that do not co-localize with Lawc and therefore are not likely to be directly affected by the lawcP1 mutation in this tissue. We examined seven genes, three found to be over-expressed and four under-expressed in the lawcP1 mutant microarray. We observed no difference in Lawc localization in ctn larvae when compared to wild-type (Figure 5B-C). Only two of the genes examined, CG9497 and ACXD, co-localize with Lawc (Figure 5B-C). These two genes are then likely to be direct targets of Lawc regulation. Since the polytene chromosomes were obtained from salivary gland preparations, qRT-PCR was performed to confirm transcript mis-expression in lawcP1 salivary glands. The amount of CG9497 transcript is reduced 87% in the mutant (α < 0.01) while ACXD is reduced 63% relative to the control (α < 0.02) (Figure 4B-C). These results are similar to the values obtained by microarray analysis (data not shown). qRT-PCR analysis of both genes revealed that they are infrequently transcribed even in the control background (data not shown). The CG32301 gene is located close to ACXD (Crosby et al. 2007) and CG32301 transcript levels are also reduced in the lawcP1 mutant (data not shown). Likewise, the only affected gene near CG9497 is CG9500 (Crosby et al. 2007), and its expression is also reduced (data not shown). No other genes located within 20 kb in either direction of CG9497 or ACXD were found to have obviously altered expression in the microarray studies. Therefore, the presence of these neighboring genes should not confound the conclusion that lawc mutants have decreased expression of some genes.
Lawc interacts with the nuclear proteasome
The results discussed so far suggest a role for the Lawc protein in transcription. Examination of the sequence of this protein does not reveal the presence of conserved domains that could give insights into the function of Lawc. In order to gain a better understanding of the role of the Lawc protein in transcription, we performed a yeast-two hybrid screen to try to identify proteins that interact with Lawc. A fusion protein containing full-length Lawc and the LexA DNA-binding domain served as the bait. Yeast-two hybrid hits were chosen for sequencing after they exhibited growth on selective media, demonstrated high β-galactosidase expression, and failed to grow on selective media in the presence of only the empty LexA DNA-binding domain (Table 1).
Table 1.
Results of a yeast two-hybrid screen to identify Lawc interacting proteins.
| Hit | Predicted/known function | Frequency |
|---|---|---|
| BAP111 | Brahma remodeling complex | 1 |
| CG4857-PB | unknown | 1 |
| CG7466-PA | signal transduction | 1 |
| CG11674-PA | spliceosomal complex | 1 |
| Delta | Notch receptor ligand | 8 |
| Dlc90F | cytoskeleton | 1 |
| dREGγ | nuclear proteasome | 4 |
| Fur2 | tyrosine kinase signal transduction |
2 |
| Hsp26 | protein chaperone | 5 |
| Mob1 | unknown | 1 |
| N | signal transduction | 1 |
| Pvf1 | growth factor | 3 |
| Socs16D | cytokine mediated signaling | 2 |
| Yl | receptor-mediated endocytosis, translation |
2 |
A LexA-Lawc fusion protein was tested for the ability to interact with components of a Drosophila embryonic cDNA library. The above interactions (hits) were detected. The predicted/known function is listed as reported on Flybase (Crosby et al. 2007). Frequency refers to the number of times each protein was pulled out of the screen.
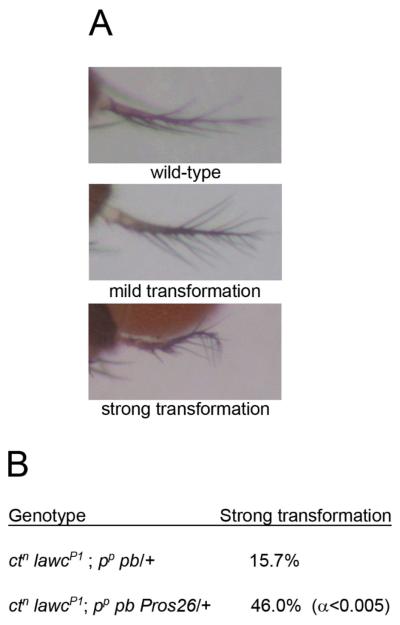
One of the proteins found to interact with Lawc is dREGγ, a component of the nuclear proteasome that was found multiple times in the screen (Table 1). In order to confirm that Lawc interacts with the REGγ proteasome in flies, we made use of a mutation in the Pros26 gene. Pros26 is a component of the 20S catalytic core (Covi et al. 1999), which is common to both the standard and REGγ proteasomes. We examined the lawc phenotype of flies hemizygous for lawcP1 and heterozygous for Pros26 pb pp and found that Pros26 pb pp significantly enhances the arista-to-leg transformation beyond that of the ctn lawcP1; pb pp/+ control (α < 0.005) (Figure 6). The Pros26 mutation does not affect the phenotype of ctn flies (data not shown). This result suggests that mutations in a proteasome component genetically interact with lawc mutations, suggesting that the two proteins act in the same pathway.
Figure 6.
Lawc interacts with the nuclear proteasome. (A) Photographs illustrate differences in the arista phenotype as scored in (B). Note that the mild transformation consists of a subtle thickening of the arista base and that the strong transformation results in a much thicker arista that is mis-shaped. (B) The α value reported is the result of a test of statistical significance between ctn lawcP1; Pros26 pp pb/+ and ctn lawcP1; pp pb/+.
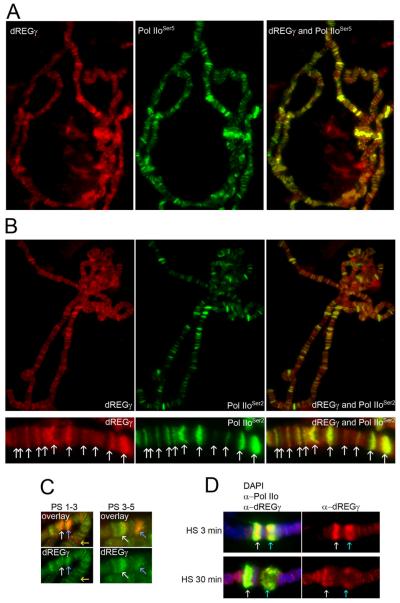
Although it has previously been reported that dREGγ is a nuclear protein, the presence of this protein on chromosomes has not been demonstrated. We find that dREGγ is also present in the interbands of polytene chromosomes and that it extensively co-localizes with Pol IIo (Figure 7). Therefore the dREGγ protein may also be involved in transcription. In addition, Lawc and dREGγ have very similar distribution patterns on polytene chromosomes. In fact, dREGγ localizes to puffs at the same puff stage as Lawc (Figure 7B), suggesting that the two proteins may play similar roles in the transcription process. However, dREGγ can also be found at these loci during the first puff stages and is recruited to actively transcribed genes before Lawc (Figure 7B). Likewise, dREGγ is present at the 87A and 87C heat-shock loci both before the arrival of Lawc and after Lawc is recruited (Figure 7C). The observed interactions between Lawc and dREGγ and the temporal order in which the two proteins are recruited to transcriptionally active genes suggest that Lawc may play a role in the regulation of the activity of the dREGγ proteasome.
Figure 7.
dREGγ has a localization pattern on polytene chromosomes similar to that of Lawc. (A) Polytene chromosomes from wild-type third instar larvae were incubated with α-dREGγ, and Pol IIoSer5. (B) Polytene chromosomes from wild-type third instar larvae were incubated with α-dREGγ, and α-Pol IIoSer2. In order to allow for a more detailed examination of α-Lawc and Pol IIoSer2 staining patterns, an enlarged and rotated version of a portion of a chromosome from each image is represented immediately below the image of the full spread. Arrows indicate regions of dREGγ-Pol IIoSer2 co-localization. (C) Polytene chromosomes from wild-type third instar larvae were incubated with α-dREGγ and α-EcR (red). The puff stage (PS) of each larva was determined. The white arrows point to 74EF and the blue arrows indicate 75B, while the yellow arrows point to 78D. (D) Images are shown with 87A on the left (white arrow) and 87C on the right (blue). Wild-type larvae were placed at 37° (HS) for the indicated amount of time. Polytene chromosomes were incubated with DAPI (blue), α-dREGγ and α-Pol IIo (green).
DISCUSSION
The lawc gene has been shown to be a member of the trxG, suggesting a function of the encoded product in chromatin-based transcriptional processes. Nevertheless, nothing is currently known about the nature of the Lawc protein or the mechanisms by which it may affect transcription. Here we show that lawc corresponds to the uncharacterized gene CG32711 and encodes a small Cys-rich protein present at sites of active transcription. Interestingly, Lawc interacts with the nuclear proteasome genetically as well as in the yeas two hybrid assay. We find that Lawc is recruited to transcribed genes after at least one round of transcription has taken place. Based on these observations, we propose a model suggesting that Lawc regulates the activity of the nuclear proteasome to control the degradation of unknown factors involved in transcription elongation. Future work should address the specific molecular mechanisms by which the Lawc protein regulates gene expression.
Based on the observation of genetic interactions between lawcP1 and a Trf2 allele, Kopytova et al. reported that lawcP1 must be a mutation in Trf2 (Kopytova et al. 2006). However, data presented here indicates that lawcP1 does not affect Trf2 transcript levels and the observed genetic interactions between lawc and Trf2 could be due to the participation of both proteins in different aspects of the transcription process. Here we demonstrate that Lawc is a nuclear protein that is present in the interbands of polytene chromosomes, where it co-localizes with active Pol II, suggesting a role for Lawc in the regulation of transcription. We find that Lawc enters chromosome loci dependent on the transcription status of the residing genes. Puff-staging data place Lawc downstream of at least one round of transcription by Pol IIo and upstream of recruitment of the SIN3 corepressor after PS 6 (Pile et al. 2000). Lawc appears to enter chromosome puffs during gene expression peaks and reduced levels of Lawc lead to excess Pol IIoSer2. Based on these data, it could be hypothesized that Lawc is involved in turning genes off, since lack of Lawc protein in lawc mutants appears to result in higher levels of elongating Pol II. Nevertheless, genes identified as targets of Lawc, such as CG9497 and ACXD, are transcriptionally down-regulated in the lawcP1 mutant background. Given these results, it is unlikely that Lawc is involved solely in repression of transcription.
An important insight in elucidating the function of Lawc is the finding that this protein interacts with the nuclear proteasome. The dREGγ component of the nuclear proteasome co-localizes with Pol IIo and is present at the same actively transcribed and 20E-responsive loci as Lawc. Although the role of the 26S proteasome in transcription is better established, there has been one study providing evidence that the REGγ proteasome can regulate transcription (Li et al. 2006). Therefore, it is possible that the effect of Lawc on transcription is mediated by the nuclear proteasome and, therefore, Lawc may regulate the degradation of an unknown component of the transcription machinery. Based on the timing of the arrival of Lawc to actively transcribed puffs, at least one round of transcription may have occurred before Lawc recruitment. Since Lawc appears to enter actively transcribed genes after Pol IIoSer2 phosphorylation during the first round of transcription, Lawc may not be required for any events prior to the generation of Pol IIoSer2. However, at the same time, mutations in the lawc gene result in an increase of Pol IIoSer2 levels. These results could be explained if Lawc is not involved in the first elongation event, but in subsequent events after the pioneering Pol IIoSer2 has crossed the template. Since this pioneering Pol IIoSer2 and its associated elongation factors have altered the chromatin environment (reviewed in Reinberg et al. 2006), it is possible that this chromatin change is responsible for the recruitment of the Lawc protein, either directly or indirectly. It has been demonstrated that highly transcribed genes do not rely heavily on the reestablishment of chromatin, presumably because Pol IIoSer2 density is so high that cryptic promoters are hidden from Pol IIa (Li et al. 2007). Perhaps new chromatin components, such as histone H3.3, are recruited to these genes but they do not have a chance to become stably incorporated into the template until transcription slows. It is possible that Lawc would be recruited to these genes at this point. The model predicts that Lawc would be most abundant at genes with a low Pol IIoSer2 density and this is consistent with the observation that Lawc intensity does not correlate with that of Pol IIo in polytene chromosomes. In addition, genes affected in the lawcP1 mutant (ACXD and CG9497) are normally expressed at low levels while unaffected genes (Trf2, CG11190, Hsp70 and Act5C genes) are highly expressed. Additional work will be required to provide answers to these questions.
ACKNOWLEDGEMENTS
We would like to thank Ellen M. Baxter for the establishment of transgenic flies and Dr. Mark Van Doren and members of his lab for providing a welcoming and stimulating environment to carry out some of the work described in the manuscript. We also thank Dr. Patrick Young for α-dREGγ, Dr. Susan M. Parkhurst for a cDNA library and Drs Allan C. Spradling, Mark Van Doren, Kyle W. Cunningham, Maya Capelson, Ellen M. Baxter, and Reed S. Shabman for advice. The EcR antibody developed by C. Thummel and D. Hogness was obtained from the Developmental Studies Hybridoma Bank established under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. This work was supported by National Science Foundation Award MCB-0618972.
REFERENCES
- Ashburner M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila. VI. Induction by ecdysone in salivary glands of D. melanogaster cultured in vitro. Chromosoma. 1972;38:255–281. doi: 10.1007/BF00290925. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Puffs, genes, and hormones revisited. Cell. 1990;61:1–3. doi: 10.1016/0092-8674(90)90205-s. [DOI] [PubMed] [Google Scholar]
- Bai J, Uehara Y, Montell DJ. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103:1047–1058. doi: 10.1016/s0092-8674(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Capelson M, Corces VG. The ubiquitin ligase dTopors directs the nuclear organization of a chromatin insulator. Mol Cell. 2005;20:105–116. doi: 10.1016/j.molcel.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Covi JA, Belote JM, Mykles DL. Subunit compositions and catalytic properties of proteasomes from developmental temperature- sensitive mutants of Drosophila melanogaster. Arch Biochem Biophys. 1999;368:85–97. doi: 10.1006/abbi.1999.1294. [DOI] [PubMed] [Google Scholar]
- Crosby MA, Goodman JL, Strelets VB, Zhang P, Gelbart WM. FlyBase: genomes by the dozen. Nucleic Acids Res. 2007;35:D486–491. doi: 10.1093/nar/gkl827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis AP, Lonard DM, Nawaz Z, O'Malley BW. Inhibition of the 26S proteasome blocks progesterone receptor-dependent transcription through failed recruitment of RNA polymerase II. The Journal of Steroid Biochemistry and Molecular Biology. 2005;94:337–346. doi: 10.1016/j.jsbmb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Dubiel W, Pratt G, Ferrell K, Rechsteiner M. Purification of an 11 S regulator of the multicatalytic protease. J Biol Chem. 1992;267:22369–22377. [PubMed] [Google Scholar]
- Dworniczak B, Seidel R, Pongs O. Puffing activities and binding of ecdysteroid to polytene chromosomes of Drosophila melanogaster. Embo J. 1983;2:1323–1330. doi: 10.1002/j.1460-2075.1983.tb01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdous A, Gonzalez F, Sun L, Kodadek T, Johnston SA. The 19S Regulatory Particle of the Proteasome Is Required for Efficient Transcription Elongation by RNA Polymerase II. Molecular Cell. 2001;7:981–991. doi: 10.1016/s1097-2765(01)00250-7. [DOI] [PubMed] [Google Scholar]
- Gillette TG, Gonzalez F, Delahodde A, Johnston SA, Kodadek T. Physical and functional association of RNA polymerase II and the proteasome. Proc Natl Acad Sci U S A. 2004;101:5904–5909. doi: 10.1073/pnas.0305411101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F, Delahodde A, Kodadek T, Johnston SA. Recruitment of a 19S proteasome subcomplex to an activated promoter. Science. 2002;296:548–550. doi: 10.1126/science.1069490. [DOI] [PubMed] [Google Scholar]
- Ho CK, Shuman S. Distinct roles for CTD Ser-2 and Ser-5 phosphorylation in the recruitment and allosteric activation of mammalian mRNA capping enzyme. Mol Cell. 1999;3:405–411. doi: 10.1016/s1097-2765(00)80468-2. [DOI] [PubMed] [Google Scholar]
- Huet F, Ruiz C, Richards G. Puffs and PCR: the in vivo dynamics of early gene expression during ecdysone responses in Drosophila. Development. 1993;118:613–627. doi: 10.1242/dev.118.2.613. [DOI] [PubMed] [Google Scholar]
- Ivaldi MS, Karam CS, Corces VG. Phosphorylation of histone H3 at Ser10 facilitates RNA polymerase II release from promoter-proximal pausing in Drosophila. Genes Dev. 2007;21:2818–2831. doi: 10.1101/gad.1604007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim FD, Thummel CS. Temporal coordination of regulatory gene expression by the steroid hormone ecdysone. Embo J. 1992;11:4083–4093. doi: 10.1002/j.1460-2075.1992.tb05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Jones K, Thummel CS. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet. 2005;6:311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- Kinyamu HK, Chen J, Archer TK. Linking the ubiquitin-proteasome pathway to chromatin remodeling/modification by nuclear receptors. J Mol Endocrinol. 2005;34:281–297. doi: 10.1677/jme.1.01680. [DOI] [PubMed] [Google Scholar]
- Kopytova DV, Krasnov AN, Kopantceva MR, Nabirochkina EN, Nikolenko JV, Maksimenko O, Kurshakova MM, Lebedeva LA, Yerokhin MM, Simonova OB, et al. Two isoforms of Drosophila TRF2 are involved in embryonic development, premeiotic chromatin condensation, and proper differentiation of germ cells of both sexes. Mol Cell Biol. 2006;26:7492–7505. doi: 10.1128/MCB.00349-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Gogol M, Carey M, Pattenden SG, Seidel C, Workman JL. Infrequently transcribed long genes depend on the Set2/Rpd3S pathway for accurate transcription. Genes Dev. 2007;21:1422–1430. doi: 10.1101/gad.1539307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lonard DM, Jung SY, Malovannaya A, Feng Q, Qin J, Tsai SY, Tsai MJ, O'Malley BW. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell. 2006;124:381–392. doi: 10.1016/j.cell.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Lu H, Flores O, Weinmann R, Reinberg D. The nonphosphorylated form of RNA polymerase II preferentially associates with the preinitiation complex. Proc Natl Acad Sci U S A. 1991;88:10004–10008. doi: 10.1073/pnas.88.22.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zawel L, Fisher L, Egly JM, Reinberg D. Human general transcription factor IIH phosphorylates the C-terminal domain of RNA polymerase II. Nature. 1992;358:641–645. doi: 10.1038/358641a0. [DOI] [PubMed] [Google Scholar]
- Marshall NF, Peng J, Xie Z, Price DH. Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J Biol Chem. 1996;271:27176–27183. doi: 10.1074/jbc.271.43.27176. [DOI] [PubMed] [Google Scholar]
- Masson P, Andersson O, Petersen UM, Young P. Identification and characterization of a Drosophila nuclear proteasome regulator. A homolog of human 11 S REGgamma (PA28gamma) J Biol Chem. 2001;276:1383–1390. doi: 10.1074/jbc.M007379200. [DOI] [PubMed] [Google Scholar]
- Pile LA, Wassarman DA. Chromosomal localization links the SIN3-RPD3 complex to the regulation of chromatin condensation, histone acetylation and gene expression. Embo J. 2000;19:6131–6140. doi: 10.1093/emboj/19.22.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. Cyclic, Proteasome-Mediated Turnover of Unliganded and Liganded ER[alpha] on Responsive Promoters Is an Integral Feature of Estrogen Signaling. Molecular Cell. 2003;11:695–707. doi: 10.1016/s1097-2765(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Reinberg D, Sims RJ., 3rd de FACTo nucleosome dynamics. J Biol Chem. 2006;281:23297–23301. doi: 10.1074/jbc.R600007200. [DOI] [PubMed] [Google Scholar]
- Schaeffer L, Roy R, Humbert S, Moncollin V, Vermeulen W, Hoeijmakers JH, Chambon P, Egly JM. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993;260:58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Schroeder SC, Schwer B, Shuman S, Bentley D. Dynamic association of capping enzymes with transcribing RNA polymerase II. Genes Dev. 2000;14:2435–2440. doi: 10.1101/gad.836300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BE, Larochelle S, Suter B, Lis JT. Cdk7 is required for full activation of Drosophila heat shock genes and RNA polymerase II phosphorylation in vivo. Mol Cell Biol. 2003;23:6876–6886. doi: 10.1128/MCB.23.19.6876-6886.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot WS, Swyryd EA, Hogness DS. Drosophila tissues with different metamorphic responses to ecdysone express different ecdysone receptor isoforms. Cell. 1993;73:1323–1337. doi: 10.1016/0092-8674(93)90359-x. [DOI] [PubMed] [Google Scholar]
- Wilk S, Chen WE, Magnusson RP. Properties of the nuclear proteasome activator PA28gamma (REGgamma) Arch Biochem Biophys. 2000;383:265–271. doi: 10.1006/abbi.2000.2086. [DOI] [PubMed] [Google Scholar]
- Wu CH, Lee C, Fan R, Smith MJ, Yamaguchi Y, Handa H, Gilmour DS. Molecular characterization of Drosophila NELF. Nucleic Acids Res. 2005;33:1269–1279. doi: 10.1093/nar/gki274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Takagi T, Wada T, Yano K, Furuya A, Sugimoto S, Hasegawa J, Handa H. NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell. 1999;97:41–51. doi: 10.1016/s0092-8674(00)80713-8. [DOI] [PubMed] [Google Scholar]
- Yao J, Munson KM, Webb WW, Lis JT. Dynamics of heat shock factor association with native gene loci in living cells. Nature. 2006;442:1050–1053. doi: 10.1038/nature05025. [DOI] [PubMed] [Google Scholar]
- Yao TP, Forman BM, Jiang Z, Cherbas L, Chen JD, McKeown M, Cherbas P, Evans RM. Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature. 1993;366:476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]
- Yao TP, Segraves WA, Oro AE, McKeown M, Evans RM. Drosophila ultraspiracle modulates ecdysone receptor function via heterodimer formation. Cell. 1992;71:63–72. doi: 10.1016/0092-8674(92)90266-f. [DOI] [PubMed] [Google Scholar]
- Zhao YM, Chen X, Sun H, Yuan ZG, Ren GL, Li XX, Lu J, Huang BQ. Effects of histone deacetylase inhibitors on transcriptional regulation of the hsp70 gene in Drosophila. Cell Res. 2006;16:566–576. doi: 10.1038/sj.cr.7310074. [DOI] [PubMed] [Google Scholar]
- Zorin ID, Gerasimova TI, Corces VG. The lawc gene is a new member of the trithorax-group that affects the function of the gypsy insulator of Drosophila. Genetics. 1999;152:1045–1055. doi: 10.1093/genetics/152.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]