Figure 4.
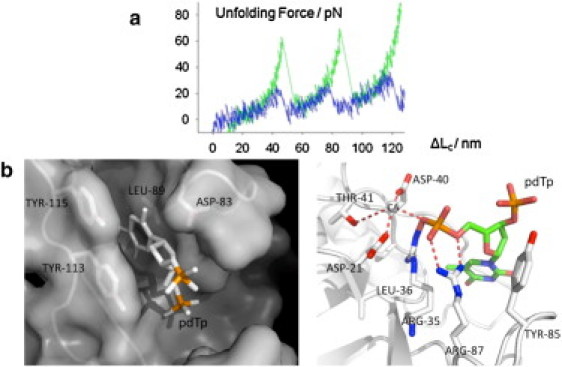
(a) A comparison of typical force-extension unfolding traces of SNase domains without (low force peak) and with (high force peak) pdTp present. (b) Detail of active site in SNase-pdTp-Ca2+ complex from PDB ID 2SNS, drawn by PyMOL (DeLano Scientific, South San Francisco, CA). The surface model shows that the thymine ring of pdTp fits well into a hydrophobic pocket formed by four major residues (left). (Solid dotted lines) Hydrogen bonds or metal interactions between pdTp and Ca2+ and the residues around the active site (right) (4).
