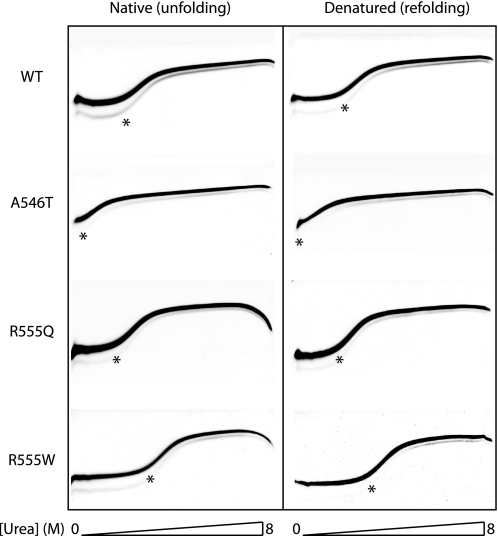
FIGURE 4.
Unfolding and refolding TUG gels of FAS1-4 variants. Chemical denaturation of FAS1-4 variants was analyzed by TUG gel electrophoresis for WT, A546T, R555Q, and R555W FAS1-4. Proteins were loaded on the TUG gels in either native buffer (left panel) or 8 m urea (right panel). The gels show rapid two-state unfolding kinetics for all FAS1-4 variants. The A546T variant is significantly less stable than the WT domain. The stability of mutant R555Q is slightly less than that of WT. Mutant R555W displays an increased stability compared with the WT FAS1-4 domain. The 0–8 m urea gradient is shown below the TUG gels.