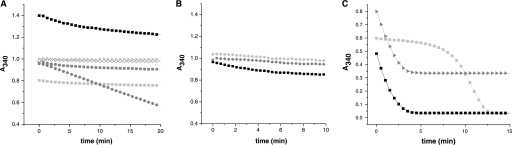
FIGURE 6.
GSNO reduction by TGR and Trxs. A, the ability of TGRWT and Grx-less TGR to reduce GSNO was tested using NADPH as an electron donor and following NADPH oxidation (A340 decrease). For TGRWT, different GSNO and enzyme concentrations were assayed as follows: 2 nm TGRWT and 250 μm (light gray box), 500 μm (dark gray box), and 1 mm (black box) GSNO concentrations, and 40 nm TGRWT and 500 μm GSNO (dark gray circle). For Grx-less TGR, 2 nm enzyme and 500 μm GSNO were used (light gray right triangle). A reaction mixture without enzyme and containing 500 μm GSNO was run for comparison (white diamond). The initial NADPH concentration was 100 μm in all cases. Note that as both reduced NADPH and GSNO contribute to A340, initial absorbance values vary according to GSNO concentrations. A marginal GSNO reduction activity is observed only at high TGRWT. B, TGRWT at 2 nm (black box), and Grx-less TGR (2 nm) plus 10 μm cTrx (dark gray circle) or 10 μm mTrx (light gray circle) were compared for GSNO reduction at 500 μm GSNO and 100 μm NADPH. No significant activity was found for either Trx. C, the effect of GSNO addition on the GR activity of TGRWT was studied by comparing the time courses for NADPH oxidation (A340 decrease) by wild-type TGR (5 nm) with GSSG 100 μm in the absence (black box) or presence (dark gray right triangle) of 500 μm GSNO. The time course of TGRWT (5 nm) at 1 mm GSSG is included as a control of an inhibited TGR (light gray box). The results indicated that at the conditions assayed TGR is not inhibited by GSNO. Note that the differences in initial absorbance values for the reaction mixtures containing different GSNO concentrations are due to the contribution of GSNO to A340.