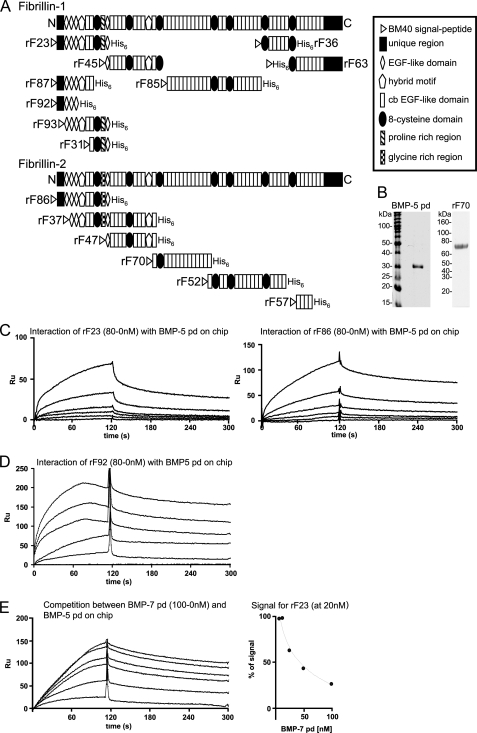
FIGURE 1.
BMP-5 pd interacts with N-terminal polypeptides of fibrillin-1 and -2. A, schematic representation of the recombinant fibrillin polypeptides used in this study. B, Coomassie Blue-stained gel of the bacterially expressed, purified, and alkylated BMP-5 pd, after SDS-PAGE in a nonreducing 12.5% gel, demonstrates purity of the sample. In addition, a Coomassie Blue-stained gel of the newly described fibrillin-2 polypeptide rF70 is shown. C, selected sensorgrams from SPR experiments with immobilized BMP-5 pd and soluble ligands rF23 or rF86 show interactions. The fibrillin polypeptides where diluted from 80 to 0 nm in HBS-EP buffer. D, SPR sensorgram with immobilized BMP-5 pd and titrated concentrations of rF92. E, BMP-5 and BMP-7 pds compete for the same binding site in fibrillin-1. Left, sensorgram shows concentration-dependent decrease of the BMP-5 pd-rF23 interaction signal, when BMP-7 pd is preincubated with rF23. BMP-5 pd was immobilized, and the fibrillin-1 N-terminal polypeptide rF23 was injected at a constant concentration of 20 nm in the presence of increasing concentrations (0–100 nm) of BMP-7 pd. Right, inhibition curve shows competition of the BMP-7 pd for the BMP-5 pd-binding site in fibrillin-1. The signal in RU obtained for rF23 at 20 nm without competitor was set as 100%. The decrease of the 100% rF23 signal was graphed against the inhibitor concentration to determine the inhibition constant (I50) for the competition reaction. To account for variations of the rF23 signal because of the buffer change caused by the addition of different amounts of competitor, a buffer matched control sensorgram without competitor was generated for each BMP-7 concentration. This control signal served as the 100% reference signal for each competitor concentration.