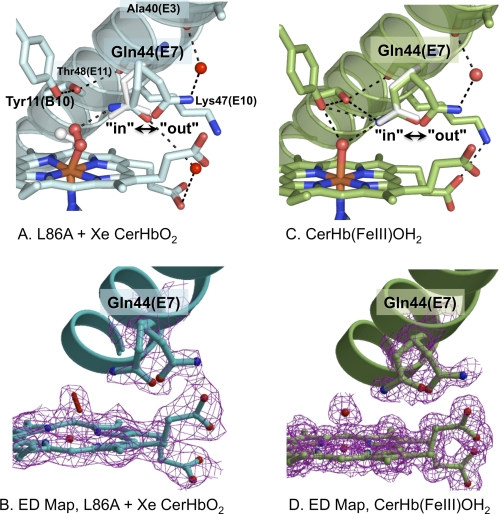
FIGURE 2.
Multiple conformations of Gln-44(E7) in various structures of CerHb. A, distal pocket, E-helix, multiple conformations of Gln-44(E7), and bound O2 of xenon-bound L86A CerHbO2 (2xkh, cyan). B, the electron density map (2Fo − Fc map contoured at 1σ) for xenon-bound L86A CerHbO2 is shown in magenta for the double conformation of Glu-44(E7), the heme group, and the oxygen molecule coordinated to the iron atom and for the Gln-44(E7) side chain refined in two (in and out) conformations. C, heme pocket, E-helix, in and out conformers of Gln-44(E7), and coordinated water of wt aquomet CerHb (2xki, green). D, electron density map (2Fo − Fc map contoured at 1σ) for wt aquomet CerHb are shown in magenta for the heme group, the heme-coordinated water molecule, and for the Gln-44(E7) side chain refined in two (in and out) conformations. In the upper panels A and C, the Gln-44(E7) side chain conformer from wt CerHbO2 (1kr7) is superimposed as white sticks. The position of bound O2 in wt CerHbO2 is also shown in panel A as white sticks. Residues Tyr-11(B10), Gln-44(E7), Lys-47(E10), and Thr-48(E11) are represented as sticks; heme-bound O2 and coordinated H2O are represented as red sticks; the heme group is displayed as sticks with the carbon atoms either light blue (panel A) or green (panel B), the iron atom in orange, and nitrogen and oxygen atoms in blue and red, respectively; external water molecules are displayed as red spheres; hydrogen bonds are drawn as dashed black lines.