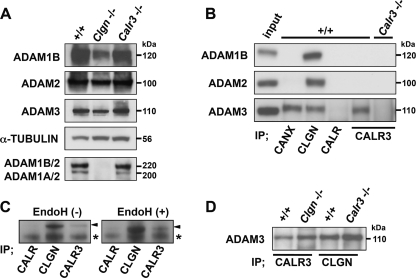
FIGURE 4.
Interactions between ADAM proteins and ER chaperones. A, Western blot analysis of testis lysates separated by SDS-PAGE under reducing conditions (upper four panels) and nonreducing conditions (lower panel). Comparable amounts of ADAM1B, ADAM2, and ADAM3 were detected in +/+, Clgn−/−, and Calr3−/− testes under reducing conditions. When ADAM1A/ADAM2 and ADAM1B/ADAM2 heterodimers were probed by anti-ADAM2 antibody under nonreducing conditions (22–24), ADAM1/ADAM2 heterodimerization was absent in Clgn−/− testis but was not impaired in Calr3−/− testis. B, immunoprecipitates (IP) with anti-CANX, anti-CALR, anti-CLGN, and anti-CALR3 antibodies from testis lysates (100 μg) were probed with the indicated antibodies. Testis lysates (10 μg) were loaded as the input control. Whereas CLGN associated with ADAM1B, ADAM2, and ADAM3, CALR3 associated with only ADAM3. C, testis lysates digested with endoglycosidase H (EndoH) were immunoprecipitated with the indicated antibodies and then probed with anti-ADAM3 antibody. Endoglycosidase H treatment changed the molecular mass of ADAM3 but did not affect the association with CLGN and CALR3. Arrowheads indicate ADAM3. Asterisks indicate the nonspecific signal. D, testis lysates from Clgn−/− and Calr3−/− mice were immunoprecipitated with anti-CALR3 and anti-CLGN antibodies and then probed with anti-ADAM3 antibody.