FIGURE 2.
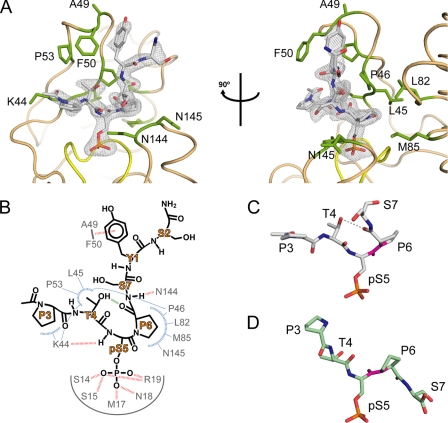
Binding of cis-proline pCTD substrate by Ssu72. A, orthogonal views of pCTD conformation and binding. The enzyme structure is shown in tan with yellow highlighting for the active site loop, and residues that form the substrate recognition surface are labeled and shown as green sticks. The gray mesh represents the Fo − Fc omit map density (contoured at 3.0σ) surrounding the substrate. B, schematic of the Ssu72-pCTD interaction. Hydrogen bonds and van der Waal contacts are shown as red and blue dashed lines, respectively. The green line represents an intramolecular hydrogen bond in the pCTD substrate. C and D, examples of cis and trans substrate configurations. The conformations of Ser(P)5 CTD peptides in complex with dSsu72 (C) and phosphatase Scp1 (D) are shown with the Ser(P)5–Pro6 peptide bond highlighted in magenta. For clarity, only residues Pro3 to Ser7 are shown. The cis isomer is stabilized by an intramolecular hydrogen bond (gray dashed line) and causes a severe kink in the CTD backbone.